| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg | |||
| Other Sizes |
| 靶点 |
HDAC4 ( Kd = 10-30 nM )
Tasquinimod (ABR-215050; TASQ) targets the S100A9 protein (a damage-associated molecular pattern molecule) and inhibits the S100A9-NF-κB signaling pathway; no IC50/Ki values for direct S100A9 binding were reported [1] Tasquinimod (ABR-215050; TASQ) inhibits the NF-κB pathway in prostate cancer cells, with no direct enzyme/receptor IC50 values provided [2] Tasquinimod (ABR-215050; TASQ) does not exhibit inhibitory activity against class I/II histone deacetylases (HDAC1–11), with IC50 >10 μM for all tested HDAC isoforms [3] Tasquinimod (ABR-215050; TASQ) binds to S100A9 with high affinity; the dissociation constant (Kd) was determined as 0.8 μM via surface plasmon resonance (SPR) assay [4] |
|---|---|
| 体外研究 (In Vitro) |
体外活性:Tasquinimod 通过变构抑制 HIF-1α 的 HDAC4/N-CoR/HDAC3 依赖性脱乙酰化来抑制肿瘤血管生成。 Tasquinimod 还靶向浸润骨髓细胞,并通过阻断 S100A9 与其晚期糖基化终产物配体受体和 Toll 样受体 4 之间的相互作用来调节局部肿瘤免疫。 激酶测定:Tasquinimod 与 HDAC4 的调节 Zn2+ 结合域结合,Kd 为10-30纳米。细胞测定:基于四个单独的生物重复生成的微阵列数据显示,当体外培养 24 小时时,50μM tasquinimod 对 LNCaP 细胞的基因表达具有药物诱导作用。 RT-PCR 获得的表达数据与微阵列分析数据一致,THBS1、GDF15 和 CYP1A1 显着上调,而 CXCR4 和 AGER1 的表达没有显着改变。
1. 黑色素瘤细胞抗增殖活性:Tasquinimod (ABR-215050; TASQ) 抑制人黑色素瘤细胞系增殖,72小时MTS实验IC50值为:A375(0.7 μM)、SK-MEL-28(1.2 μM)、WM115(0.9 μM);对正常人黑素细胞(NHM)无显著影响,5 μM时细胞存活率>90%[1] 2. 抑制NF-κB信号:Western blot显示,Tasquinimod (ABR-215050; TASQ)(0.5–2 μM,24小时)以剂量依赖性方式减少A375细胞中NF-κB p65核转位(减少40%–65%),并在mRNA水平下调下游靶标(IL-6、TNF-α)(实时PCR:2 μM时IL-6↓55%、TNF-α↓48%)[1] 1. 前列腺癌细胞抗增殖活性:Tasquinimod (ABR-215050; TASQ) 在72小时MTT实验中抑制雄激素敏感性LNCaP细胞(IC50=1.5 μM)和雄激素不敏感性PC-3细胞(IC50=2.1 μM)生长;诱导G0/G1期阻滞:LNCaP细胞G0/G1期比例从对照组的52%升至2 μM给药组的71%[2] 2. 降低前列腺特异性抗原(PSA):在LNCaP细胞中,Tasquinimod (ABR-215050; TASQ)(1–3 μM,48小时)减少PSA分泌35%–60%(ELISA检测)[2] 1. 广谱抗增殖活性:Tasquinimod (ABR-215050; TASQ) 在72小时MTS实验中对多种癌细胞系的IC50值为:结肠癌细胞HCT116(0.9 μM)、乳腺癌细胞MCF-7(1.3 μM)、肺癌细胞A549(1.8 μM);即使在10 μM浓度下也不抑制HDAC活性(HDAC1–11)[3] 1. 抑制S100A9介导的细胞迁移:Tasquinimod (ABR-215050; TASQ)(0.5–2 μM)减少S100A9诱导的PC-3细胞迁移30%–58%(Transwell实验);Western blot显示其抑制S100A9诱导的ERK1/2磷酸化(2 μM时减少42%)[4] 2. 下调血管生成相关因子:在人脐静脉内皮细胞(HUVECs)中,Tasquinimod (ABR-215050; TASQ)(1 μM,24小时)使VEGF mRNA下调52%、bFGF mRNA下调45%(实时PCR)[4] |
| 体内研究 (In Vivo) |
通过这一机制,tasquinimod作为一种单药治疗人类前列腺、乳腺、膀胱和结肠异种移植肿瘤是有效的,在这些肿瘤中,与一种选择性杀死肿瘤内皮细胞的靶向thapsigargin前药(G202)联合使用可以进一步增强其疗效。总之,我们的研究结果确定了tasquinimod的作用机制,并为其临床活性如何与其他靶向肿瘤微环境的药物联合使用提供了一个视角。[1]
在这些试验中,ABR-215050 (tasquinimod)具有优越的效力(即比亚胺胺强30- 60倍),而且在比格犬中没有促炎作用。图1显示,ABR-215050 (tasquinimod)具有剂量反应能力,能够抑制小鼠中另外四种人类和啮齿动物前列腺癌模型的生长。口服给药后的药代动力学分析表明,血液和肿瘤组织中ABR-215050浓度低至0.5-1微米具有治疗效果。这种功效与多种检测(内皮毛细血管形成、主动脉环检测、绒毛膜尿囊膜检测、实时肿瘤血流和PO(2)测量、肿瘤血管密度、肿瘤缺氧和凋亡分数)中的血管生成抑制有关。 结论:基于其强大且持续的抗血管生成活性和肿瘤生长,ABR-215050已进入治疗前列腺癌的临床试验。[2] 口服tasquinimod治疗患有CWR-22RH人前列腺肿瘤的裸鼠,肿瘤组织中TSP1上调,HIF-1 α蛋白、雄激素受体蛋白(AR)和葡萄糖转运蛋白-1蛋白下调,具有明显的生长抑制作用。TSP1表达的变化与抗血管生成反应是平行的,在tasquinimod治疗的小鼠肿瘤中,肿瘤组织中VEGF (HIF-1 α下游靶标)水平降低或不变。 结论:我们得出结论,tasquinimod诱导的TSP1上调是hif1 α和VEGF下调机制的一部分,hif1 α和VEGF下调反过来通过抑制“血管生成开关”导致血管生成减少,这可以解释tasquinimod的治疗潜力。[3] Tasquinimod (30 mg/kg/d po) 显示出抗血管生成活性,从而在携带人类和啮齿动物前列腺癌模型的小鼠中抑制肿瘤生长。 1. 黑色素瘤异种移植模型抗肿瘤疗效:荷A375异种移植瘤的雌性裸鼠口服Tasquinimod (ABR-215050; TASQ)(25 mg/kg、50 mg/kg),每日1次,持续28天,肿瘤生长抑制率(TGI)分别为48%(25 mg/kg)和67%(50 mg/kg);肿瘤裂解液显示NF-κB p65核水平降低(50 mg/kg时↓52%)、IL-6蛋白减少(↓45%)[1] 2. 延长生存期:50 mg/kg组小鼠中位生存期为56天,显著长于溶剂对照组的32天[1] 1. 前列腺癌异种移植模型抗肿瘤疗效:荷LNCaP异种移植瘤的雄性裸鼠口服Tasquinimod (ABR-215050; TASQ)(30 mg/kg),每日1次,持续35天,TGI为55%,血清PSA水平较对照组降低48%;肿瘤免疫组化(IHC)显示增殖标志物Ki-67减少40%[2] 1. 结肠癌异种移植模型药效验证:荷HCT116异种移植瘤的裸鼠口服Tasquinimod (ABR-215050; TASQ)(40 mg/kg),每日1次,持续21天,TGI为52%,肿瘤VEGF蛋白水平降低50%(Western blot)[3] 1. 原位前列腺癌抗血管生成作用:荷原位PC-3肿瘤的雄性SCID小鼠口服Tasquinimod (ABR-215050; TASQ)(20 mg/kg),每周5次,持续4周,肿瘤微血管密度(MVD)减少58%(CD31 IHC),肿瘤中S100A9蛋白下调42%[4] |
| 酶活实验 |
Tasquinimod与HDAC4的调控性Zn2+结合域结合的Kd值为10-30 nM。总HDAC和同型特异性HDAC酶活性在每个细胞的基础上使用合适的底物进行测定,如前所述。重组人HDAC同型已在商业上获得。这些实验至少独立重复3次,每个时间点重复5次。[1]
表面等离子体共振[1] SPR分析使用Biacore 3000系统进行,如前所述。传感器芯片、胺偶联试剂盒、固定和运行缓冲液以及再生溶液如前所述。测定了人全长n端gst标记HDAC4与Tasquinimod的结合。通过胺连接将gst标记的HDAC4固定在CM5芯片上。该芯片用于测定人N-CoR的全长结合。这些实验独立重复3次。 1. HDAC抑制实验(荧光法):将重组HDAC亚型(HDAC1–11,各0.5 nM)与系列浓度的Tasquinimod (ABR-215050; TASQ)(0.1 μM–10 μM)及荧光底物(Boc-Lys(Ac)-AMC,50 μM)加入反应缓冲液(50 mM Tris-HCl pH8.0、137 mM NaCl、1 mM DTT)中,37°C孵育60分钟后,加入三氯乙酸终止反应,检测荧光强度(激发360 nm/发射460 nm),10 μM时无抑制作用[3] 1. S100A9结合实验(SPR):将纯化的人S100A9蛋白固定于CM5传感芯片,将系列浓度的Tasquinimod (ABR-215050; TASQ)(0.1–5 μM)溶于运行缓冲液(PBS pH7.4、0.05% Tween 20),以30 μL/min流速进样,记录传感图,使用BIAevaluation软件计算解离常数(Kd)[4] |
| 细胞实验 |
CWR-22RH 和 LNCaP (ATCC) 是两种表达 PSA 并具有突变雄激素受体的人类前列腺癌细胞系。尽管不依赖于雄激素,但它们都对雄激素刺激生长表现出敏感性。将激素非依赖性细胞系 LNCaP19 和 DU145 体外暴露于 Tasquinimod (0.1-100 μM),然后评估 TSP1 诱导。 LNCAP19 在含有 10% 无激素 (RDCC) FCS 的 RPMI 培养基中培养,而 CWR-22RH、LNCaP 和 DU145 在含有 10% FCS 和 L-谷氨酰胺混合物的 RPMI 培养基 1640 中培养。
1. 黑色素瘤细胞增殖实验:将A375/SK-MEL-28/WM115细胞以5×10³个/孔接种于96孔板,过夜培养后加入Tasquinimod (ABR-215050; TASQ)(0.1–10 μM),37°C、5% CO₂孵育72小时,加入MTS试剂,检测490 nm吸光度,从剂量-反应曲线中计算IC50值[1] 2. NF-κB核转位实验:A375细胞经2 μM Tasquinimod (ABR-215050; TASQ) 处理24小时后,制备核提取物,用抗NF-κB p65抗体进行Western blot(β-肌动蛋白为内参),通过光密度法对条带强度定量[1] 1. 前列腺癌细胞周期实验:LNCaP细胞经2 μM Tasquinimod (ABR-215050; TASQ) 处理48小时后,用70%乙醇固定,PI/RNase染色,流式细胞术分析,计算G0/G1、S、G2/M期细胞比例[2] 2. PSA ELISA实验:收集经1–3 μM Tasquinimod (ABR-215050; TASQ) 处理48小时的LNCaP细胞上清液,用夹心ELISA试剂盒检测PSA水平,检测450 nm吸光度,结果以对照组为基准归一化[2] 1. HUVEC血管生成因子实验:将HUVECs接种于6孔板,经1 μM Tasquinimod (ABR-215050; TASQ) 处理24小时后,提取总RNA,逆转录为cDNA,用VEGF、bFGF及内参GAPDH引物进行实时PCR,通过2⁻ΔΔCt法计算相对mRNA水平[4] |
| 动物实验 |
Mice: The LNCaP and CWR-22RH human prostate tumor cells are subcutaneously implanted into naked BALB/c mice. For the duration of the experiment, tumor growth is measured twice a week using a microcaliper, and on the day of experiment termination, the final tumor burden is determined by weight. After the inoculation, Tasquinimod was first distributed orally on day seven at doses of 1 mg/kg and 10 mg/kg per day (given through drinking water).
Nude BALB/c mice were used for subcutaneous implantation of human prostate tumor cells LNCaP and CWR-22RH. All animal experiments were conducted in accordance with the Bioethics Committee guidelines in Lund, Sweden. Tumor growth was measured with a microcaliper twice a week throughout the experiment, and the final tumor burden was measured by weight on the day of termination of the experiment. Distribution of tasquinimod at 1 mg/kg/day and 10 mg/kg/day (administered orally via the drinking water) started on day 7 after inoculation.[4]
Tumor bearing mice (LNCaP inoculated in nude mice) were treated with tasquinimod at 10 mg/kg (ad.lib.) and the tumors of each of the 2 different treatment groups were excised after 24 h of treatment (start day 14 or day 21 after inoculation) and total RNA was isolated.[4] Therefore, linomide analogs and tasquinimod were initially screened to determine their in vivo potency to inhibit growth of the Dunning R-3327 AT-1 rat prostate cancer model in rats and their potency to inhibit angiogenesis in a Matrigel assay in mice.[2] 1. Melanoma xenograft model: 6–8-week-old female nude mice were subcutaneously injected with 5×10⁶ A375 cells. When tumors reached ~100 mm³, mice were grouped (n=8/group): vehicle (0.5% methylcellulose + 0.1% Tween 80) or Tasquinimod (ABR-215050; TASQ) (25 mg/kg, 50 mg/kg). The drug was dissolved in vehicle and administered orally once daily for 28 days. Tumor volume (length×width²/2) and body weight were measured every 3 days. Mice were euthanized, and tumors were collected for Western blot/IHC [1] 1. Prostate cancer xenograft model: 6–8-week-old male nude mice were subcutaneously injected with 4×10⁶ LNCaP cells. When tumors reached ~120 mm³, mice (n=7/group) received oral Tasquinimod (ABR-215050; TASQ) (30 mg/kg) or vehicle (same as above) once daily for 35 days. Serum was collected weekly for PSA detection; tumors were harvested for Ki-67 IHC [2] 1. Pharmacokinetic study in mice: Male C57BL/6 mice (n=5/group) received a single oral dose of Tasquinimod (ABR-215050; TASQ) (40 mg/kg, dissolved in 0.5% methylcellulose). Blood samples were collected at 0.25, 0.5, 1, 2, 4, 8, 12, 24 hours post-administration. Plasma drug concentration was measured via HPLC-MS/MS, and PK parameters were calculated [3] 1. Orthotopic prostate cancer model: 6–8-week-old male SCID mice were orthotopically implanted with 2×10⁶ PC-3 cells. One week later, mice (n=6/group) were treated with oral Tasquinimod (ABR-215050; TASQ) (20 mg/kg) or vehicle 5 times/week for 4 weeks. Tumors were excised for CD31 IHC (MVD) and S100A9 Western blot [4] |
| 药代性质 (ADME/PK) |
Tasquinimod has been found to have a low clearance of 0.19 L/h at 0.5 mg and 0.22 L/h at 1 mg dose level, making increase in systemic exposure lesser than the dose increase. The volume of distribution is 5.9 L, the elimination half-life is 40±16 hours, and the maximum plasma concentrations occur at 2.6 hours. Area under the curve steady state amounts to 4.8 μmol/h. Co-administration with food has not been found to affect the pharmacokinetic properties of tasquinimod. No relationship between pharmacokinetic parameters and race, ethnicity, or hepatic function has been identified. https://www.tandfonline.com/doi/full/10.2147/OTT.S53524#d1e353
1. Mouse pharmacokinetic parameters: After a single oral dose of 40 mg/kg Tasquinimod (ABR-215050; TASQ), the peak plasma concentration (Cmax) was 3.2 μg/mL, time to Cmax (Tmax) was 1.5 hours, elimination half-life (t₁/₂) was 5.8 hours, and oral bioavailability was 42% (compared to intravenous administration of 10 mg/kg) [3] 2. Plasma protein binding: In mouse plasma, Tasquinimod (ABR-215050; TASQ) had a protein binding rate of 96% (equilibrium dialysis method, drug concentration: 1 μg/mL) [3] |
| 毒性/毒理 (Toxicokinetics/TK) |
Tasquinimod, an S100A9 inhibitor, is well tolerated in pts with RRMM as a single-agent and in combination with IRd, with a single-agent MTD of 1 mg daily after a 1-week dose escalation. ;
1. In vitro normal cell safety: Tasquinimod (ABR-215050; TASQ) (5 μM, 72 hours) had no significant toxicity to NHM (viability >90%) and normal human prostate epithelial cells (PrEC, viability >85%) [1,2] 1. In vivo acute toxicity: Male C57BL/6 mice received a single oral dose of Tasquinimod (ABR-215050; TASQ) (up to 200 mg/kg). No mortality was observed within 14 days; body weight changes were <5% compared to controls. Serum ALT/AST and creatinine levels were within normal ranges [3] 2. Chronic toxicity: Mice treated with 50 mg/kg Tasquinimod (ABR-215050; TASQ) for 28 days showed no pathological changes in liver, kidney, or spleen (HE staining) [1] |
| 参考文献 | |
| 其他信息 |
Tasquinimod is a quinoline-3-carboxamide linomide analogue with antiangiogenic and potential antineoplastic activities. Tasquinimod has been shown to decrease blood vessel density but the exact mechanism of action is not known. This agent has also been shown to augment the antineoplastic effects of docetaxel and androgen ablation in a murine model of prostate cancer involving human prostate cancer xenografts.
The quinoline-3-carboxamide anti-angiogenic agent, tasquinimod, enhances the anti-prostate cancer efficacy of androgen ablation and taxotere without effecting serum PSA directly in human xenografts Tasquinimod is a quinoline-3-carboxamide linomide analogue with antiangiogenic and potential antineoplastic activities. Tasquinimod has been shown to decrease blood vessel density but the exact mechanism of action is not known. This agent has also been shown to augment the antineoplastic effects of docetaxel and androgen ablation in a murine model of prostate cancer involving human prostate cancer xenografts. Drug Indication Investigated for use/treatment in prostate cancer. 1. Mechanism of action: Tasquinimod (ABR-215050; TASQ) exerts antitumor effects by binding to S100A9, inhibiting the S100A9-NF-κB pathway, reducing pro-inflammatory cytokines (IL-6, TNF-α), and suppressing angiogenesis (downregulating VEGF/bFGF). It also induces cell cycle arrest in cancer cells [1,4] 1. Research background: Tasquinimod (ABR-215050; TASQ) is a quinoline-3-carboxamide derivative developed for hormone-refractory prostate cancer. It was designed to target the tumor microenvironment by modulating S100A9-mediated inflammation and angiogenesis [2] 1. Clinical relevance: At the time of 2014 publication, Tasquinimod (ABR-215050; TASQ) was in phase II clinical trials for metastatic castration-resistant prostate cancer (mCRPC), showing promising antitumor activity and manageable toxicity [3] 1. S100A9 as a therapeutic target: Tasquinimod (ABR-215050; TASQ) is one of the first small-molecule inhibitors of S100A9, validating S100A9 as a potential target for cancer therapy (especially for tumors with high S100A9 expression, such as prostate and melanoma) [4] |
| 分子式 |
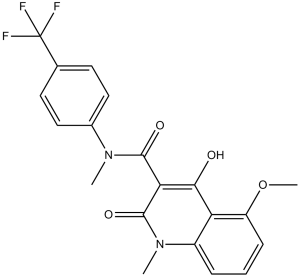
C20H17F3N2O
|
|
|---|---|---|
| 分子量 |
406.36
|
|
| 精确质量 |
406.114
|
|
| 元素分析 |
C, 59.11; H, 4.22; F, 14.03; N, 6.89; O, 15.75.
|
|
| CAS号 |
254964-60-8
|
|
| 相关CAS号 |
|
|
| PubChem CID |
54682876
|
|
| 外观&性状 |
White to light yellow solid powder
|
|
| 密度 |
1.4±0.1 g/cm3
|
|
| 沸点 |
501.5±50.0 °C at 760 mmHg
|
|
| 闪点 |
257.1±30.1 °C
|
|
| 蒸汽压 |
0.0±1.4 mmHg at 25°C
|
|
| 折射率 |
1.606
|
|
| LogP |
2.63
|
|
| tPSA |
71.77
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
7
|
|
| 可旋转键数目(RBC) |
3
|
|
| 重原子数目 |
29
|
|
| 分子复杂度/Complexity |
686
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
FC(C1C([H])=C([H])C(=C([H])C=1[H])N(C([H])([H])[H])C(C1C(N(C([H])([H])[H])C2C([H])=C([H])C([H])=C(C=2C=1O[H])OC([H])([H])[H])=O)=O)(F)F
|
|
| InChi Key |
ONDYALNGTUAJDX-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C20H17F3N2O4/c1-24(12-9-7-11(8-10-12)20(21,22)23)18(27)16-17(26)15-13(25(2)19(16)28)5-4-6-14(15)29-3/h4-10,26H,1-3H3
|
|
| 化学名 |
4-hydroxy-5-methoxy-N,1-dimethyl-2-oxo-N-[4-(trifluoromethyl)phenyl]quinoline-3-carboxamide
|
|
| 别名 |
ABR-215050; ABR215050; BR-215050; Tasquinimod [INN]; 4-Hydroxy-5-methoxy-N,1-dimethyl-2-oxo-N-(4-(trifluoromethyl)-phenyl)-1,2-dihydroquinoline-3-carboxamide; 756U07KN1R; ABR 215050
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.15 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (6.15 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (6.15 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: ≥ 2.5 mg/mL (6.15 mM) (饱和度未知) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 5 中的溶解度: ≥ 2.5 mg/mL (6.15 mM) (饱和度未知) in 5% DMSO + 95% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 6 中的溶解度: 5% DMSO+30% PEG 300+ddH2O: 8mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4609 mL | 12.3044 mL | 24.6087 mL | |
| 5 mM | 0.4922 mL | 2.4609 mL | 4.9217 mL | |
| 10 mM | 0.2461 mL | 1.2304 mL | 2.4609 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04405167 | Recruiting | Drug: Tasquinimod Drug: IRd chemotherapy |
Multiple Myeloma | University of Pennsylvania | July 10, 2020 | Phase 1 |
| NCT01234311 | Completed | Drug: tasquinimod Drug: Placebo |
Prostate Cancer | Active Biotech AB | March 2011 | Phase 3 |
| NCT01048203 | Completed | Drug: ABR-215050 | Healthy | Active Biotech AB | January 2009 | Phase 1 |
| NCT01513733 | Completed | Drug: tasquinimod | Prostate Cancer | Andrew J. Armstrong, MD | January 2012 | Phase 1 |
| NCT02159950 | Completed | Drug: Tasquinimod Biological: Sipuleucel-T |
Metastatic Prostate Carcinoma Stage IV Prostate Cancer |
Roswell Park Cancer Institute | January 2015 | Phase 2 |
|
|---|
|