| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 靶点 |
Endogenous Metabolite; Microbial Metabolite
|
|---|---|
| 体外研究 (In Vitro) |
在 HBeAg 阳性 CHB 患者分离的 PBMC 中,牛磺胆酸(100 μM,24 小时)可降低 CD3+CD8+ T 和 NK 细胞的百分比[2]。牛磺胆酸(100 μM,24 小时)可降低 CD3+CD8+ T 和 NK 细胞中 IFN-α 驱动的细胞因子和细胞毒性颗粒水平(IFN-γ、TNF-α、颗粒酶 B)[2]。
牛磺胆酸(TCA)在体外抑制IFN-α的免疫调节活性[2] 鉴于IFN-α是重要的免疫调节剂[33],且我们的研究结果表明牛磺胆酸(TCA)不仅抑制慢性乙型肝炎(CHB)患者对IFN-α治疗的应答反应,还在体外和体内实验中抑制CD3+CD8+ T细胞与NK细胞的效应功能(图2-5),我们推测TCA可能通过抑制IFN-α的免疫调节活性来削弱其功能。由于缺乏合适的HBeAg阳性CHB动物模型[34],我们采用HBeAg阳性CHB患者新鲜分离的外周血单个核细胞(PBMCs)进行实验。将细胞分别用IFN-α或TCA联合IFN-α刺激24小时后,通过流式细胞术检测CD3+CD8+ T细胞和NK细胞内IFN-γ、TNF-α、颗粒酶B及穿孔素的表达水平。结果显示,与对照组相比,IFN-α刺激显著提升了这些效应分子水平(图6),这与既往研究[35,36]一致;而TCA联合IFN-α组则较单独IFN-α组表现出明显的效应分子表达下降(图6)。这些结果充分证明,牛磺胆酸(TCA)在体外能显著抑制IFN-α的免疫调节作用。 体外实验证实,牛磺胆酸(TCA)可通过促进胆管细胞分泌VEGF-A来刺激其增殖——该效应可被渥曼青霉素阻断,且VEGFR-2激酶抑制剂能有效抑制这种促增殖作用。[3] |
| 体内研究 (In Vivo) |
当rAAV8-1.3HBV注射到C57BL/6小鼠尾静脉时,牛磺胆酸(口服灌胃,100mg/kg,2周)可以通过降低NK和CD3+CD8+T细胞的比例来增加HBV复制[2] 。通过上调 VEGF-A 表达,牛磺胆酸(饮食中 1%,1 周)可保护肝动脉结扎 (HAL) 引起的胆管细胞损伤 [3]。
本研究发现脂多糖(LPS)和环孢素A(CsA)可分别上调和下调TNF-α与IL-1β的基因及蛋白表达。牛磺胆酸(TCA)(0.25g/kg、0.125g/kg)能恢复被抑制的TNF-α和IL-1β表达,并提高CD4(+)/CD8(+)比值。体外实验中,TCA(15μg/mL)可抑制TNF-α和IL-1β的过度产生;TCA(0.15μg/mL-15μg/mL)能抑制IL-1β和TNF-α基因表达的异常升高;而TCA(0.15μg/mL)则可恢复被抑制的TNF-α和IL-1β表达水平。 结论:牛磺胆酸(TCA)的免疫调节功能可能通过调控TNF-α和IL-1β的基因与蛋白表达,以及提升CD4(+)/CD8(+) T细胞比例来实现。[1] 牛磺胆酸(TCA)在体内损害CD3+CD8+ T细胞和NK细胞的效应功能[2] 为验证TCA是否在体内抑制CD3+CD8+ T细胞和NK细胞的效应功能,我们在尾静脉注射rAAV8-1.3HBV病毒6周后,对C57BL/6小鼠进行为期2周的TCA灌胃(100mg/kg/天)或对照饮食处理(图5A)。灌胃后血清TCA水平显著升高(图S6)。研究发现TCA处理显著降低了NK和CD3+CD8+ T细胞比例(图5B)。此外,与对照组相比,TCA处理组小鼠的CD8+ T细胞和NK细胞产生的细胞因子及细胞毒性颗粒水平更低(图5C、D)。重要的是,TCA处理组小鼠血清HBsAg、HBeAg和HBV DNA水平均高于对照组(图5E)。这些结果表明,TCA通过降低CD3+CD8+ T细胞和NK细胞比例并损害其效应功能来促进HBV复制。 在胆管结扎(BDL)联合肝动脉结扎(HAL)大鼠模型中,长期饲喂牛磺胆酸(TCA)可预防HAL引起的胆管丢失和胆管细胞分泌功能下降。牛磺胆酸(TCA)还能阻止HAL诱导的肝组织VEGF-A和VEGFR-2表达降低以及循环VEGF-A水平下降,这些保护作用可被渥曼青霉素预处理所阻断。[3] |
| 细胞实验 |
脾淋巴细胞上清液及总RNA制备[1]
将脾淋巴细胞悬浮于含3 mM L-谷氨酰胺、10 mM HEPES缓冲液、100 U/mL青霉素-链霉素及10%胎牛血清(FBS)的RPMI-1640培养基中,调整细胞浓度为1×10⁶个/mL,接种至六孔培养板(2 mL/孔),分别加入LPS(终浓度10 μg/mL)或CsA(终浓度0.01 μg/mL)。实验随机分为6组:对照组(正常小鼠淋巴细胞)、LPS/CsA组(仅含LPS/CsA);其余4组分别加入不同浓度牛磺胆酸(TCA)(0.015 μg/mL、0.15 μg/mL、1.5 μg/mL和15 μg/mL)。培养48小时后,采用相应方法制备淋巴细胞上清液和总RNA。 体外细胞培养与刺激[2] 新鲜分离的人外周血单个核细胞(PBMCs)接种于96孔板,在37℃、5% CO2条件下培养。细胞分为三组:空白培养基组、IFN-α(1000 U/ml)组、IFN-α(1000 U/ml)联合牛磺胆酸(TCA)(100 μM)组,处理24小时。随后用佛波醇12-肉豆蔻酸酯13-乙酸酯(PMA)和离子霉素刺激5小时,通过流式细胞术检测NK细胞和CD8+ T细胞内IFN-γ、TNF-α、颗粒酶B及穿孔素的表达。 牛磺胆酸盐介导NRIC增殖中VEGF-A分泌的作用评估[3] 胰酶消化后,将NRIC以每孔10,000个细胞接种于96孔板(每孔200 μL培养基)。实验设置:空白对照组、牛磺胆酸(20 μM)组、渥曼青霉素(100 nM)预处理1小时后牛磺胆酸刺激组、VEGFR-2激酶抑制剂I(100 nM)预处理1小时后牛磺胆酸刺激组,处理48小时。采用CellTiter 96 AQueous One Solution细胞增殖检测试剂盒评估NRIC增殖情况,于490 nm波长测定吸光度。数据以处理组相对于对照组的倍数变化表示。为验证NRIC上清液对胆管细胞增殖的差异性刺激作用(取决于上清液中VEGF含量),我们分别用BSA或20 μM牛磺胆酸(TCA)处理NRIC 24小时获取上清液(后者含更高水平VEGF-A),在渥曼青霉素(100 nM)预处理1小时或不预处理条件下,通过PCNA免疫印迹检测细胞生长情况。 |
| 动物实验 |
Animal/Disease Models: C57BL/6 mice[2]
Doses: 100-mg/kg Route of Administration: po (oral gavage), for 2 weeks after tail vein injection with rAAV8-1.3HBV for 6 weeks Experimental Results: decreased the percentage of NK and CD3+CD8+ T cells . Increases serum HBsAg, HBeAg, and HBV DNA levels. Taurocholic acid (TCA) dissociated and depurated [1] Fresh bovine and/or sheep galls were collected from a slaughterhouse. The bile was deproteinated using alcohol after filtered by filter paper, and then it was condensed using rotary evaporator after depigmented by activated carbon. Crude bile acids were obtained after salting out, extracting and dewatering. Taurocholic acid (TCA) was dissociated and depurated from crude bile acid by chromatography techniques and the purity was detected by high performance liquid chromatography. Its purity was > 98.7%. Kunming mice (half male and half female), weight 20 ± 2 g, were obtained from the experimental center, Inner Mongolia University. All animals were maintained at a controlled temperature (22 ± 2 °C), and a regular light/dark cycle (7:00 am–7:00 pm, light) and all animals had free access to food and water. The animals were divided into 7 groups of 8 each (Table 1). All animals were treated orally by administration of intra-gastric gavage (i.g.) once daily and sacrificed after 7 days of treatment. Peripheral blood, serum and spleen were prepared for flow cytometry, ELISA and mRNA extraction respectively. Establishment of a recombinant adeno-associated virus type 8 (rAAV8)-mediated HBV replication mouse model [2] rAAV8 carrying the 1.3-mer wild-type HBV genome (rAAV8-1.3HBV) was used to establish an immunocompetent mouse model for chronic HBV infection.27 A total of 5 × 1010 viral genomes/200 μl virus were injected into the tail vein of each C57BL/6 mouse. The mice were bled every other week to monitor the HBsAg, HBeAg, and HBV DNA levels. After 6 weeks, mice were fed by oral gavage for 2 weeks with either 100-mg/kg Taurocholic acid (TCA) daily or a control diet. Following this, the mice were sacrificed. Male Fischer 344 rats (150 to 175 gm) were kept in a temperature-controlled environment (22°C) with a 12-hour light-dark cycle and fed ad libitum rat chow. The studies were performed in: (i) BDL (for isolation of cells) or bile duct incannulated (BDI, for bile collection) rats that (immediately after BDL or BDI) were fed bile acid control diet or 1% taurocholic acid diet (which represents an approximate dose of 275 μmol/day) for 1 week; (ii) rats that (immediately after BDL or BDI + HAL) were fed bile acid control diet or 1% taurocholic acid diet; and (iii) rats that (immediately after BDL or BDI + HAL) were fed 1% Taurocholic acid (TCA) for 1 week in the presence of daily injections of 0.9% NaCl or wortmannin (0.7 mg/kg body weight). The groups of animals used in the study are summarized in Table 1. Since we have previously shown that daily injections of wortmannin or DMSO (in which wortmannin is dissolved) to BDL or BDI rats do not affect cholangiocyte apoptosis, proliferation and functional activity, these groups of animals were not included in the study. BDL, BDI and HAL were performed as described. Before each procedure, animals were anesthetized with sodium pentobarbital (50 mg/kg body weight, IP).[3] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Transported by carrier-mediated processes bidirectionally across mammalian proximal tubule. After secretion into the biliary tract, bile acids are largely (95%) reabsobed in the intestine (mainly in the terminal ileum), returned to the liver, and then again secreted in bile (enterohepatic circulation). The disposition kinetics of [(3)H]taurocholate ([(3)H]TC) in perfused normal and cholestatic rat livers were studied using the multiple indicator dilution technique and several physiologically based pharmacokinetic models. The serum biochemistry levels, the outflow profiles and biliary recovery of [(3)H]TC were measured in three experimental groups: (i) control; (ii) 17 alpha-ethynylestradiol (EE)-treated (low dose); and (iii) EE-treated (high dose) rats. EE treatment caused cholestasis in a dose-dependent manner. A hepatobiliary TC transport model, which recognizes capillary mixing, active cellular uptake, and active efflux into bile and plasma described the disposition of [(3)H]TC in the normal and cholestatic livers better than the other pharmacokinetic models. An estimated five- and 18-fold decrease in biliary elimination rate constant, 1.7- and 2.7-fold increase in hepatocyte to plasma efflux rate constant, and 1.8- and 2.8-fold decrease in [(3)H]TC biliary recovery ratio was found in moderate and severe cholestasis, respectively, relative to normal. There were good correlations between the predicted and observed pharmacokinetic parameters of [(3)H]TC based on liver pathophysiology (e.g. serum bilirubin level and biliary excretion of [(3)H]TC). In conclusion, these results show that altered hepatic /taurocholate/ pharmacokinetics in cholestatic rat livers can be correlated with the relevant changes in liver pathophysiology in cholestasis. It has been reported that the adjuvant-induced inflammation could affect drug metabolism in liver. /The authors/ further investigated the effect of inflammation on drug transport in liver using taurocholate as a model drug. The hepatic disposition kinetics of [(3)H]taurocholate in perfused normal and adjuvant-treated rat livers were investigated by the multiple indicator dilution technique and data were analyzed by a previously reported hepatobiliary taurocholate transport model. Real-time RT-PCR was also performed to determine the mRNA expression of liver bile salt transporters in normal and diseased livers. The uptake and biliary excretion of taurocholate were impaired in the adjuvant-treated rats as shown by decreased influx rate constant k(in) (0.65 +/- 0.09 vs. 2.12 +/- 0.30) and elimination rate constant k(be) (0.09 +/- 0.02 vs. 0.17 +/- 0.04) compared with control rat group, whereas the efflux rate constant k(out) was greatly increased (0.07 +/- 0.02 vs. 0.02 +/- 0.01). The changes of mRNA expression of liver bile salt transporters were found in adjuvant-treated rats. Hepatic taurocholate extraction ratio in adjuvant-treated rats (0.86 +/- 0.05, n = 6) was significantly reduced compared with 0.93 +/- 0.05 (n = 6) in normal rats. Hepatic extraction was well correlated with altered hepatic ATP content (r(2) = 0.90). In conclusion, systemic inflammation greatly affects hepatic ATP content/production and associated transporter activities and causes an impairment of transporter-mediated solute trafficking and pharmacokinetics. For more Absorption, Distribution and Excretion (Complete) data for TAUROCHOLIC ACID (6 total), please visit the HSDB record page. Metabolism / Metabolites Taurocholic acid has known human metabolites that include 2-[[(4R)-4-[(3R,5R,7R,10S,12S,13R)-7,12-Dihydroxy-10,13-dimethyl-3-sulfooxy-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoyl]amino]ethanesulfonic acid. |
| 毒性/毒理 (Toxicokinetics/TK) |
Interactions
Sitosterol & taurocholate given together to rats inhibited cholesterol 7alpha-hydroxylase activity. Chickens receiving taurocholate iv did not show active tubular excretion; however, it inhibited tubular excretioN of phenolsulfonphthaleiN & of n-methylnicotinamide. In the anesthetized rat, the low incidence of erosions with indomethacin was markedly increased by concurrent gastric perfusion with acid saline & taurocholate. When a combination of aspirin & taurocholic acid was introduced to 8 subjects the mean electrical potential difference also fell significantly from 38.6 1.8 mv to 17.9 1.8 mv, but mean duration of this change (27 min) was significantly longer than found after individual admin. For more Interactions (Complete) data for TAUROCHOLIC ACID (14 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Mice ip 620 mg/kg LD50 Rat ip 450 mg/kg |
| 参考文献 |
|
| 其他信息 |
Taurocholic acid is a bile acid taurine conjugate of cholic acid that usually occurs as the sodium salt of bile in mammals. It has a role as a human metabolite. It is an amino sulfonic acid and a bile acid taurine conjugate. It is functionally related to a cholic acid. It is a conjugate acid of a taurocholate.
The product of conjugation of cholic acid with taurine. Its sodium salt is the chief ingredient of the bile of carnivorous animals. It acts as a detergent to solubilize fats for absorption and is itself absorbed. It is used as a cholagogue and cholerectic. Taurocholic acid is a metabolite found in or produced by Escherichia coli (strain K12, MG1655). Taurocholic acid has been reported in Ursus thibetanus, Homo sapiens, and other organisms with data available. taurocholic acid is a metabolite found in or produced by Saccharomyces cerevisiae. The product of conjugation of cholic acid with taurine. Its sodium salt is the chief ingredient of the bile of carnivorous animals. It acts as a detergent to solubilize fats for absorption and is itself absorbed. It is used as a cholagogue and cholerectic. Therapeutic Uses Cholagogues and Choleretics; Detergents Dried bile from the Himalayan bear (Yutan) has been used for centuries in China to treat liver disease. /Bile/ |
| 分子式 |
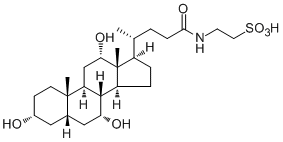
C₃₀H₅₃NO₇S
|
|---|---|
| 分子量 |
571.81
|
| 精确质量 |
515.291
|
| CAS号 |
81-24-3
|
| 相关CAS号 |
145-42-6 (mono-hydrochloride salt)
|
| PubChem CID |
6675
|
| 外观&性状 |
Clusters of slender, four-sided prisms from alcohol + ether
Crystals |
| 密度 |
1.265g/cm3
|
| 熔点 |
125°C (rough estimate)
|
| 折射率 |
1.565
|
| LogP |
3.839
|
| tPSA |
152.54
|
| 氢键供体(HBD)数目 |
5
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
35
|
| 分子复杂度/Complexity |
891
|
| 定义原子立体中心数目 |
11
|
| SMILES |
C[C@H](CCC(NCCS(=O)(O)=O)=O)[C@@]1(C)CC[C@@]2(C)[C@]3(C)[C@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@]3(C)C[C@H](O)[C@]12C
|
| InChi Key |
WBWWGRHZICKQGZ-HZAMXZRMSA-N
|
| InChi Code |
InChI=1S/C26H45NO7S/c1-15(4-7-23(31)27-10-11-35(32,33)34)18-5-6-19-24-20(14-22(30)26(18,19)3)25(2)9-8-17(28)12-16(25)13-21(24)29/h15-22,24,28-30H,4-14H2,1-3H3,(H,27,31)(H,32,33,34)/t15-,16+,17-,18-,19+,20+,21-,22+,24+,25+,26-/m1/s1
|
| 化学名 |
2-[[(4R)-4-[(3R,5S,7R,8R,9S,10S,12S,13R,14S,17R)-3,7,12-trihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoyl]amino]ethanesulfonic acid
|
| 别名 |
Taurocholate; TAUROCHOLIC ACID; Taurocholate; 81-24-3; Cholaic acid; Cholyltaurine; N-Choloyltaurine; Cholic acid taurine conjugate; Taurine, N-choloyl-; Cholyltaurine; N-Choloyl taurine
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~193.91 mM)
H2O : ~100 mg/mL (~193.91 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (4.85 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (4.85 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (4.85 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7488 mL | 8.7442 mL | 17.4883 mL | |
| 5 mM | 0.3498 mL | 1.7488 mL | 3.4977 mL | |
| 10 mM | 0.1749 mL | 0.8744 mL | 1.7488 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。