| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 500mg |
|
||
| 1g |
|

| 靶点 |
Bile acid derivative; Microbial Metabolite
|
|---|---|
| 体外研究 (In Vitro) |
在健康个体中,牛磺脱氧胆酸的中位钠浓度为 33.9 nM [1]。牛去氧胆酸抑制 N-3H-甲基东莨菪碱与 M3 毒蕈碱摄取的结合,IC50 为 170 μM [1]。 Tauodeoxycholate (0.05–1.00 mM; 1-6) 促进肠上皮细胞的生长 [2]。当牛脱氧胆酸盐(0.05–1.00 mM;24 小时)导致细胞周期 S 期浓度显着增加和 G1 期浓度显着降低时,c 细胞增殖测试就会增加 [2]。
IEC-6或Caco-2细胞用不同浓度的Taurodeoxychloic Acid/牛磺脱氧胆酸(0.05至1 mmol/L)处理,并测定增殖情况。通过DNA断裂分析和核染色来测量细胞凋亡。用碘化丙啶流式细胞术测定细胞相。通过Northern和Western印迹分析确定C-myc表达,特异性C-myc反义抑制C-myc功能。 结果:细胞结构无变化。未诱导细胞凋亡。暴露于牛磺脱氧胆酸六天后,IEC-6细胞增殖显著增加。流式细胞术显示细胞周期的S期浓度显著增加,G1期浓度显著降低。牛磺脱氧胆酸还可增加c-myc蛋白和mRNA的表达,抑制c-myc功能可阻止牛磺脱氧胆碱诱导的细胞增殖。 结论:暴露于生理浓度的胆汁盐牛磺脱氧胆酸会增加肠上皮细胞的增殖。这种作用至少部分是通过c-myc依赖机制介导的。胆汁盐对肠粘膜有有益的影响[1]。 |
| 体内研究 (In Vivo) |
接受牛去氧胆酸(0.5 mg/kg;注射;一次)治疗的已证实败血症的小鼠受到保护[1],但 TGR5 KO 小鼠则不然。
TDCA/牛磺脱氧胆酸对脓毒症小鼠具有保护作用[1] 当我们在注射LPS后30分钟或24小时静脉注射牛磺脱氧胆酸/TDCA(0.5mg/kg)时,分别有80%和50%的小鼠存活(图1A,p<0.05)。0.4 mg/kg的TDCA剂量足以获得这种效果(图S1A)。此外,在盲肠结扎和穿刺后2小时输注TDCA时,70%的小鼠存活(CLP,图1B,p<0.05)。静脉输注(1 mg/kg)后,TDCA的血浆Cmax(=502 ng/ml)约为之前报告的50%溶血浓度(420μg/ml)的1/1000(图S1B),比体外细胞毒性剂量低约1/1000(33)。TDCA在脓毒症下对TGR5 KO小鼠没有保护作用(图S1C)。 TDCA/牛磺脱氧胆酸可减少脓毒症小鼠的肝肾损伤(图1C、D和图S2)。肾脏的H&E染色显示,注射LPS的小鼠表现出明显的小管空泡变性(图1C中的箭头)。TDCA输注几乎完全改善了LPS诱导的肾脏损伤(图1C)。肾小球毛细血管环基底膜和肾小管上皮上的粘多糖也用PAS染色(图1C右栏中的箭头)。LPS+PBS组刷状边界的丧失非常显著(箭头),TDCA输注后显著恢复(图1C中的箭头)。TDCA输注使LPS注射后4小时的肾功能、肝功能和低血压恢复正常(图1D、E、图S2)。在LPS注射和CLP模型中,TDCA也显著抑制了TNF-α、MCP-1、IL-6和IL-1β等细胞因子的产生(图1F-H,图S3)。 牛磺脱氧胆酸/TDCA可增加CD11B+Gr1hi细胞的表型[1] 如前所述(34),脓毒症小鼠在注射LPS后48小时和CLP后72小时的脾细胞数量减少(图2A、图S4)。然而,在LPS注射后48小时,LPS+TDCA/牛磺酸组的脾细胞总数显著增加,CLP+TDCA组在72小时时脾细胞总数也显著增加。CD11b+Gr1+细胞在LPS+TDCA组和CLP+TTCA组中均增加(图2B、C、图S4、S5)。TDCA治疗没有增加T细胞或CD11c+细胞的数量(图S6)。CD4+FoxP3+Treg细胞的数量或这些细胞上CTLA4的表达没有显著变化(图S7)。 胆汁酸(BA)通过与多种受体相互作用来控制代谢和炎症。在这里,我们报告静脉注射牛磺脱氧胆酸盐(TDCA)可降低血清促炎细胞因子,使低血压正常化,保护肾脏免受损伤,并延长脓毒症期间小鼠的存活时间牛磺脱氧胆酸/TDCA增加了脓毒症小鼠脾脏中粒细胞-髓系衍生抑制细胞(MDSCLT)的数量,这些细胞与未经TDCA治疗的MDSC不同。FACS分选的MDSCLT细胞抑制T细胞增殖,并在过继转移时比MDSCL更好地提供对败血症的保护。蛋白质组学分析表明,TDCA控制着MDSCLT免疫蛋白质组的染色质沉默、选择性剪接和翻译,从而增加了抑瘤素、乳铁蛋白和CD244等抗炎分子的表达。TDCA还能降低促炎分子如中性粒细胞弹性蛋白酶的表达。这些发现表明,TDCA在全球范围内编辑蛋白质组,以增加MDSCLT细胞的数量,并影响其免疫调节功能,以解决败血症期间的全身炎症。 |
| 细胞实验 |
细胞增殖测定[2]
细胞类型: IEC-6 和 caco -2 细胞 测试浓度: 0、0.05、0.50 和 1.00 mM 孵育时间:1、2、4 Myc IEC-6 细胞蛋白和 mRNA 表达 [2]。和6天 实验结果:以剂量依赖性方式显着刺激肠上皮细胞增殖。 细胞周期分析 [2] 细胞类型: IEC-6 细胞 测试浓度: 0、0.05、0.50、1.00 mM 孵育时间: 24 h 实验结果: S期细胞急剧增多,G1期细胞急剧减少。 蛋白质印迹分析[2] 细胞类型: IEC-6 细胞 测试浓度: 0.5 mM 孵育时间:1天和6天 实验结果:c-myc蛋白表达显着增加。 T细胞增殖试验[1] 使用MACS和泛T细胞分离试剂盒从小鼠脾脏中纯化T细胞。在含有10%热灭活FBS和2mM谷氨酰胺的RPMI 1640培养基中,用1μg/ml抗CD3和10μg/ml抗CD28抗体刺激总共2×105个正常脾脏T细胞。将来自LPS+PBS组或LPS+牛磺脱氧胆酸/TDCA组的FACS分选的CD11b+Gr1hi细胞与T细胞在96孔平底板中以4×104/孔(E:T=1:5)的终浓度混合,并在37°C下在加湿的5%CO2气氛中培养96小时。不含CD11b+Gr1hi细胞培养的T细胞作为阴性对照。用1μCi[3H]甲基胸苷脉冲细胞18小时。用Filtermate Harvester收获细胞,用MicroBeta平板计数器测量同位素掺入。数据表示为每分钟计数(cpm)±平均值标准误差(SEM)。 CD11b+Gr1hi细胞的过继转移[1] 在B6小鼠腹腔注射LPS后30分钟,通过尾静脉静脉输注PBS或牛磺脱氧胆酸/TDCA。LPS注射后24小时,从脾脏中分离出细胞。在冰上将细胞在阻断缓冲液中孵育30分钟后,用生物素偶联的抗小鼠Gr1抗体(克隆RB6-8C5)对细胞进行染色,然后在4°C下用抗生物素微珠染色15分钟。用MACS缓冲液(0.5%BSA和2 mM EDTA的PBS溶液)洗涤后,使用LS柱对细胞进行阳性选择。用MACS预分选的Gr1+细胞进一步用抗CD11b(与PE结合)和F4/80(与FITC结合)的单克隆抗体染色,用于FACS分选。使用流式细胞仪对CD11b+Gr1hi F4/80int细胞进行分选。共1×105个细胞通过尾静脉注射到B6小鼠体内。受体小鼠在过继转移前24小时腹腔注射LPS。 细胞裂解物的溶液内消化[1] CD11b+Gr1hi细胞是从2组小鼠(LPS+PBS和LPS+TDCA/牛磺脱氧胆酸)中分选出来的FACS。使用8M尿素缓冲液制备FACS分选的CD11b+Gr1hi脾细胞的裂解物,并通过BCA测定法测定蛋白质浓度。将二硫苏糖醇加入裂解物(3 mM)中,在室温下孵育1小时。将细胞裂解物与碘乙酰胺(5 mM)混合,在暗室中孵育1 h。将一份裂解物与10份50 mM碳酸氢铵混合,在37°C下用胰蛋白酶(1/50×细胞裂解物的总蛋白量)消化16 h。随后使用宏旋柱(C-18)对样品进行脱盐。该柱预先用80%乙腈中的0.1%三氟乙酸(TFA)活化,随后用0.1%TFA水溶液(pH<3.0)平衡。将样品装入柱中,在室温下以1000×g离心2分钟。用0.1%TFA的水溶液洗涤柱,用0.1%TFA的80%乙腈溶液洗脱肽部分。肽样品用CentriVap®台式真空浓缩器干燥,肽浓度用BCA试剂盒测定。 iTRAQ标记肽[1] 使用用于相对和绝对定量的等压标签(iTRAQ)比较2组(LPS+PBS和LPS+TDCA/牛磺脱氧胆酸)CD11b+GR1hi细胞的蛋白质组。根据iTRAQ试剂盒的制造商方案,对每组100微克肽进行标记。简而言之,肽样品用500 mM碳酸氢三乙基铵(TEAB)缓冲液复溶,超声处理并涡旋。将用乙醇溶解的4-plex iTRAQ试剂加入肽样品中,并在室温下孵育1小时。向其中一份样品中加入3份0.05%TFA,并在常温下孵育30分钟。将iTRAQ标记的肽合并,随后使用CentriVap®台式真空浓缩器浓缩至300μl。然后将样品与1ml 50mM碳酸氢三乙基铵(TEAB)混合。 |
| 动物实验 |
Animal/Disease Models: C57BL/6N mouse, lipopolysaccharide injection model of sepsis [1]
Doses: 0.5 mg/kg Route of Administration: intravenous (iv) (iv)injection 30 minutes or 24 hrs (hrs (hours)) after LPS injection Experimental Results:Improved survival rate of septic mice . diminished liver and kidney damage in septic mice. Improves systemic inflammation and normalizes blood pressure in septic mice. LPS injection model of sepsis [1] The survival rate of the female mice was determined after i.p. injection of LPS (20 mg/kg), followed by the i.v. infusion of 200 μl of PBS or Taurodeoxychloic Acid/TDCA for 20 min (0.5 mg/kg, unless otherwise indicated) using a Medfusion 2001 system at 30 min (unless otherwise indicated) after LPS injection. For the protection assay using IL-10 KO mice, 5 mg/kg LPS were injected i.p. For the adoptive transfer experiments, B6 mice were injected i.v. with 100 μl of purified cells. The mice were treated with LPS 24 h prior to adoptive transfer, unless otherwise specified. CLP-induced sepsis model [1] Female B6 mice were anesthetized, and a small abdominal midline incision was made. The cecum was ligated below the ileocecal valve and punctured 3 times using a 23-gauge needle. The abdominal incision was closed with an auto-metal clip. The same procedure was applied to the sham-operated animals, with the exception of the ligation and puncture of the cecum. The mice were subsequently infused with 200 μl of PBS or Taurodeoxychloic Acid/TDCA i.v. at 2 h after CLP. |
| 毒性/毒理 (Toxicokinetics/TK) |
Taurodeoxycholate (TDCA) inhibits various inflammatory responses suggesting potential clinical application. However, the toxicity of TDCA has not been evaluated in detail in vivo. We investigated the acute toxicity and 4-week repeated-dose toxicity of TDCA following intravenous infusion under Good Laboratory Practice regulations. In the sighting study of acute toxicity, one of two rats (one male and one female) treated with 300 mg/kg TDCA died with hepatotoxicity, suggesting that the approximate 50% lethal dose of TDCA is 300 mg/kg. Edema and discoloration were observed at the injection sites of tails when rats were infused with 150 mg/kg or higher amount of TDCA once. In 4-week repeated-dose toxicity study, no treatment-related mortality or systemic changes in hematology and serum biochemistry, organ weights, gross pathology, or histopathology were observed. However, the tail injection site showed redness, discharge, hardening, and crust formation along with histopathological changes such as ulceration, edema, fibrosis, and thrombosis when rats were infused with 20 mg/kg TDCA. Taken together, TDCA induced no systemic toxicity or macroscopic lesions at the injection site at a dose of 10 mg/kg/day, which is 33 times higher than the median effective dose observed in a mouse sepsis model. These findings suggest that TDCA might have a favorable therapeutic index in clinical applications. [5]
|
| 参考文献 |
|
| 其他信息 |
A bile salt formed in the liver by conjugation of deoxycholate with taurine, usually as the sodium salt. It is used as a cholagogue and choleretic, also industrially as a fat emulsifier.
More than 2 million individuals worldwide suffer from sepsis on an annual basis. Because a plethora of pathogenic signaling pathways are simultaneously activated in septic patients, clinical trials targeting a single inflammatory mediator, coagulation factor or pro-inflammatory signal transducer have not shown significant survival benefits. In contrast to former strategies examining the blockade of pro-inflammatory pathways, targeting intrinsic immune regulatory mechanisms may be more effective for inhibiting the broad spectrum of pathways that are activated in sepsis. For these reasons, in vivo expansion of MDSCLT using a pharmacological dose of Taurodeoxychloic Acid/TDCA may be a plausible approach to inhibit the broad-spectrum pathogenesis exhibited in septic patients.[1] This study shows that bile salt Taurodeoxychloic Acid/TDCA at least partially increases intestinal epithelial proliferation by regulating transcription of the proto-oncogene c-myc, which has been shown to play an important regulatory role during intestinal epithelial proliferation. This study further defines physiological roles for bile salts in the intestinal mucosa. Thus TDCA can affect cellular functions not just digestive functions in the intestinal mucosa and these effects occur via mediating expression of cell signaling and transcription factors. Further studies are needed to elucidate the role of bile salts during injury, repair and growth within the intestinal mucosa.[2] |
| 分子式 |
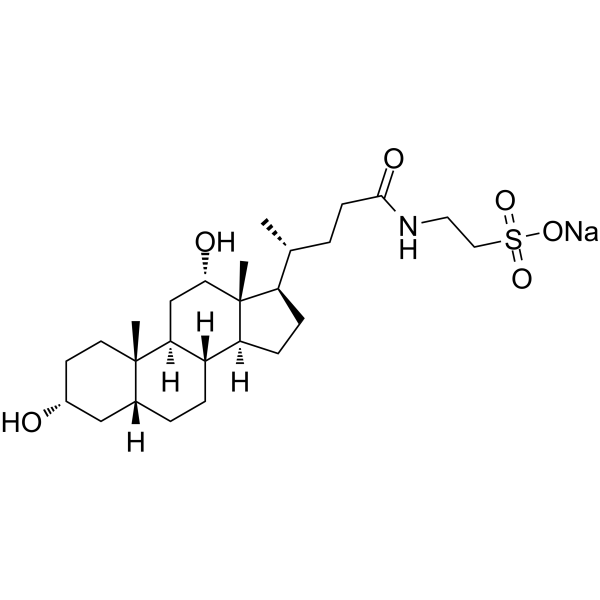
C26H44NO6S-.NA+
|
|---|---|
| 分子量 |
521.68546
|
| 精确质量 |
521.278
|
| 元素分析 |
C, 59.86; H, 8.50; N, 2.68; Na, 4.41; O, 18.40; S, 6.15
|
| CAS号 |
1180-95-6
|
| 相关CAS号 |
207737-97-1; 1180-95-6; Taurodeoxycholic acid sodium hydrate;110026-03-4;Taurodeoxycholic acid-d5;Taurodeoxycholic acid-d4 sodium;2410279-82-0;Taurodeoxycholic acid-d4;1332881-65-8
|
| PubChem CID |
23664773
|
| 外观&性状 |
White to off-white solid powder
|
| 熔点 |
168 °C (dec.)(lit.)
|
| LogP |
4.526
|
| tPSA |
135.14
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
35
|
| 分子复杂度/Complexity |
864
|
| 定义原子立体中心数目 |
10
|
| SMILES |
C[C@H](CCC(=O)NCCS(=O)(=O)[O-])[C@H]1CC[C@@H]2[C@@]1([C@H](C[C@H]3[C@H]2CC[C@H]4[C@@]3(CC[C@H](C4)O)C)O)C.[Na+]
|
| InChi Key |
YXHRQQJFKOHLAP-FVCKGWAHSA-M
|
| InChi Code |
InChI=1S/C26H45NO6S.Na/c1-16(4-9-24(30)27-12-13-34(31,32)33)20-7-8-21-19-6-5-17-14-18(28)10-11-25(17,2)22(19)15-23(29)26(20,21)3;/h16-23,28-29H,4-15H2,1-3H3,(H,27,30)(H,31,32,33);/q;+1/p-1/t16-,17-,18-,19+,20-,21+,22+,23+,25+,26-;/m1./s1
|
| 化学名 |
sodium;2-[[(4R)-4-[(3R,5R,8R,9S,10S,12S,13R,14S,17R)-3,12-dihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoyl]amino]ethanesulfonate
|
| 别名 |
Taurodeoxycholic acid sodium salt; 1180-95-6; SODIUM TAURODEOXYCHOLATE; UNII-5PE424S83L; 5PE424S83L; EINECS 214-652-0; Taurodeoxycholate, Sodium; 214-652-0;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~191.68 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (3.99 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (3.99 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (3.99 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9168 mL | 9.5842 mL | 19.1685 mL | |
| 5 mM | 0.3834 mL | 1.9168 mL | 3.8337 mL | |
| 10 mM | 0.1917 mL | 0.9584 mL | 1.9168 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。