| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
Telbivudine 通过逆转 B19V 引起的 BIRC3 失调,干扰细胞凋亡过程,防止脆弱细胞死亡[2]。
|
||
|---|---|---|---|
| 体内研究 (In Vivo) |
替比夫定是一种用于治疗乙型肝炎感染的抗病毒药物。它由瑞士制药公司诺华公司以商品名 Sebivo(欧洲)和 Tyzeka(美国)销售。临床试验表明它比拉米夫定或阿德福韦更有效,并且不太可能引起耐药性。 Telbivudine是一种合成的胸苷核苷类似物,它是胸苷的L-异构体。每天服用一次。替比夫定是一种强效抗病毒药物,可为代偿性慢性乙型肝炎患者提供有效且持续的病毒抑制。在临床试验中,替比夫定 600 mg 每天一次的治疗结果比拉米夫定 100 mg 或阿德福韦 10 mg 每天一次的治疗效果显着改善,并且替比夫定治疗的患者比拉米夫定治疗的患者的病毒耐药性显着降低。替比夫定与中等遗传耐药性障碍相关,并且由于 HBV DNA 水平检测不到的患者预后显着改善,建议在第 24 周(以及此后每月 6 周)监测 HBV DNA 水平,并添加核苷/如果存在病毒血症,可使用无交叉耐药性的核苷酸类似物(例如阿德福韦酯),以降低耐药风险(路线图概念)。在长达 4 年的临床试验中,替比夫定通常具有良好的耐受性,并且与拉米夫定具有相似的耐受性。
|
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Absorbed following oral administration. Telbivudine absorption and exposure were unaffected when a single 600–mg dose was administered with a high–fat (~55 g), high–calorie (~950 kcal) meal. Telbivudine is eliminated primarily by urinary excretion of unchanged drug. 7.6 +/- 2.9 L/h [Normal renal function (Clcr>80 mL/min)] 5.0 +/- 1.2 L/h [Mild renal function impairement (Clcr=50-80 mL/min)] 2.6 +/- 1.2 L/h [Moderate renal function impairement (Clcr=30-49 mL/min)] 0.7 +/- 0.4 L/h [Severe renal function impairement (Clcr<30 mL/min)] Metabolism / Metabolites No metabolites of telbivudine were detected following administration of [14C]–telbivudine in humans. Telbivudine is not a substrate, or inhibitor of the cytochrome P450 (CYP450) enzyme system. Biological Half-Life Approximately 15 hours. |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Telbivudine shares many features with the other L-nucleosides (lamivudine, emtricitabine) and has been linked to transient flares of hepatitis B during and after treatment of chronic hepatitis B. Serum ALT elevations above 3 times normal occurred in 5% to 10% of patients on telbivudine, which was comparable to other nucleoside analogues. Three types of flares can arise with nucleoside analogue therapy: transient and usually asymptomatic flares around the time of initiation of therapy (treatment flares), exacerbations of disease after development of antiviral resistance to telbivudine (breakthrough flares) and after stopping treatment (withdrawal flares). Cases of exacerbation of hepatitis B after development of antiviral resistance or upon telbivudine withdrawal can be severe and some cases have qualified as acute liver failure. No instances of lactic acidosis with hepatic steatosis have been reported with telbivudine therapy of hepatitis B, but isolated cases of suspected mitochondrial injury with myopathy have been reported. In trials of telbivudine therapy in pregnant women with HBsAg and HBeAg in serum and high levels of HBV DNA, therapy appeared to lessen if not eliminate maternal infant transmission, but withdrawal flares of hepatitis B occurred in some women with active disease and ALT elevations before therapy. In women with “immune tolerant” hepatitis B with high levels of HBV DNA without serum aminotransferase elevations, withdrawal flares are uncommon and levels of HBV DNA rise to previous levels on stopping therapy but without a concurrent flare of disease. Likelihood score: E (unlikely cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Telbivudine has been discontinued from the US market. It has not been studied in nursing mothers being treated for hepatitis B infection. An alternate drug may be preferred, especially while nursing a newborn or preterm infant. No difference exist in infection rates between breast-fed and formula-fed infants born to hepatitis B-infected women, as long as the infant receives hepatitis B immune globulin and hepatitis B vaccine at birth. Mothers with hepatitis B are encouraged to breastfeed their infants after their infants receive these preventative measures. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding In vitro binding of telbivudine to human plasma proteins is low (3.3%). |
||
| 参考文献 | |||
| 其他信息 |
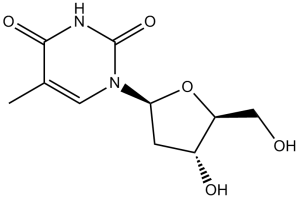
Telbivudine is a pyrimidine 2'-deoxyribonucleoside that is the L-enantiomer of thymine. A synthetic thymidine nucleoside analogue with activity against HBV DNA polymerase. It has a role as an antiviral drug and an EC 2.7.7.49 (RNA-directed DNA polymerase) inhibitor. It is functionally related to a thymine. It is an enantiomer of a thymidine.
Telbivudine is a synthetic thymidine nucleoside analog with specific activity against the hepatitis B virus. Telbivudine is orally administered, with good tolerance, lack of toxicity and no dose-limiting side effects. Telbivudine is a Hepatitis B Virus Nucleoside Analog Reverse Transcriptase Inhibitor. The mechanism of action of telbivudine is as a Nucleoside Reverse Transcriptase Inhibitor. Telbivudine is a nucleoside analogue and antiviral inhibitor of hepatitis B virus (HBV) replication which is used alone and in combination with other agents in the therapy of the hepatitis B. Telbivudine does not appear to be a significant cause of drug induced liver injury, but can be associated with flares of the underlying hepatitis B either during therapy or upon withdrawal. Telbivudine is a synthetic thymidine nucleoside analogue with antiviral activity highly specific for the treatment of hepatitis B virus (HBV). Intracellularly, telbivudine is phosphorylated to its active metabolite, telbivudine triphosphate. The dideoxy telbivudine triphosphate competes with thymidine for incorporation into viral DNA, thereby causing DNA chain termination and inhibiting the function of HBV DNA polymerase (reverse transcriptase). This results in the blockade of HBV DNA replication and viral propagation. A thymidine derivative and antiviral agent that inhibits DNA synthesis by HEPATITIS B VIRUS and is used for the treatment of CHRONIC HEPATITIS B. See also: Thymidine (annotation moved to). Drug Indication For the treatment of chronic hepatitis B in adult and adolescent patients ≥16 years of age with evidence of viral replication and either evidence of persistent elevations in serum aminotransferases (ALT or AST) or histologically active disease. Sebivo is indicated for the treatment of chronic hepatitis B in adult patients with compensated liver disease and evidence of viral replication, persistently elevated serum alanine aminotransferase (ALT) levels and histological evidence of active inflammation and/or fibrosis. Initiation of Sebivo treatment should only be considered when the use of an alternative antiviral agent with a higher genetic barrier to resistance is not available or appropriate. Treatment of chronic hepatitis B Mechanism of Action Telbivudine 5'–triphosphate inhibits HBV DNA polymerase (reverse transcriptase) by competing with the natural substrate, thymidine 5'–triphosphate. This leads to the chain termination of DNA synthesis, thereby inhibiting viral replication. Incorporation of telbivudine 5'–triphosphate into viral DNA also causes DNA chain termination, resulting in inhibition of HBV replication. Telbivudine inhibits anticompliment or second-strand DNA. Pharmacodynamics Telbivudine is a synthetic thymidine nucleoside analogue with activity against hepatitis B virus (HBV). Telbivudine is the unmodified β–L enantiomer of the naturally occurring nucleoside, thymidine. It undergoes phosphorylation via interaction with cellular kinases to form the active metabolite, telbivudine 5'-triphosphate. |
| 分子式 |
C10H14N2O5
|
|
|---|---|---|
| 分子量 |
242.23
|
|
| 精确质量 |
242.09
|
|
| CAS号 |
3424-98-4
|
|
| 相关CAS号 |
Granisetron-d3 (1-methyl-d3);1224925-76-1;Telbivudine-d4;1134182-00-5
|
|
| PubChem CID |
159269
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.5±0.1 g/cm3
|
|
| 熔点 |
188-190ºC
|
|
| 折射率 |
1.584
|
|
| LogP |
-1.11
|
|
| tPSA |
104.55
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
5
|
|
| 可旋转键数目(RBC) |
2
|
|
| 重原子数目 |
17
|
|
| 分子复杂度/Complexity |
381
|
|
| 定义原子立体中心数目 |
3
|
|
| SMILES |
CC1=CN(C(=O)NC1=O)[C@@H]2C[C@H]([C@@H](O2)CO)O
|
|
| InChi Key |
IQFYYKKMVGJFEH-CSMHCCOUSA-N
|
|
| InChi Code |
InChI=1S/C10H14N2O5/c1-5-3-12(10(16)11-9(5)15)8-2-6(14)7(4-13)17-8/h3,6-8,13-14H,2,4H2,1H3,(H,11,15,16)/t6-,7+,8+/m1/s1
|
|
| 化学名 |
1-((2S,4R,5S)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-5-methylpyrimidine-2,4(1H,3H)-dione
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 3.25 mg/mL (13.42 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 32.5 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 3.25 mg/mL (13.42 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 32.5 mg/mL 澄清 DMSO 储备液加入 900 μL 20% SBE-β-CD 生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 3.25 mg/mL (13.42 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 10 mg/mL (41.28 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.1283 mL | 20.6415 mL | 41.2831 mL | |
| 5 mM | 0.8257 mL | 4.1283 mL | 8.2566 mL | |
| 10 mM | 0.4128 mL | 2.0642 mL | 4.1283 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Renoprotective Effects of Telbivudine in Chronic Hepatitis B
CTID: NCT03778567
Phase: Phase 4 Status: Completed
Date: 2018-12-19