| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
PTGER2 ( Ki = 9.9 nM )
TG4-155 is a selective antagonist of prostaglandin E2 (PGE2) receptor EP2 (PTGER2); the Ki value for EP2 binding is 0.3 μM [3] TG4-155 exhibits an IC50 of 1.2 μM for inhibiting PGE2-induced cAMP accumulation in EP2-expressing cells, and has no significant binding affinity (Ki > 10 μM) for other prostanoid receptors (EP1, EP3, EP4, DP1, FP, IP, TP) [2][3] |
|---|---|
| 体外研究 (In Vitro) |
TG4-155 抑制血清素 5-HT2B 受体,IC50=2.6 µM,抑制 hERG(人 Ether-à-go-go-相关基因),IC50=12 µM[1]。 PGE2 (0.1-10 μM) 刺激以浓度依赖性方式显着增强人前列腺癌细胞系 PC3 细胞的生长,在大约 1 μM 时获得最大反应。 TG4-155(0.01-1μM;48 小时)以浓度依赖性方式显着抑制 PGE2 诱导的癌细胞增殖[1]。细胞活力测定[1] 细胞系:PC3 细胞 浓度:48 小时 孵育时间:0.01、0.1 和 1 μM 结果:以浓度依赖性方式显着抑制 PGE2 诱导的癌细胞增殖。
1. 在原代大鼠皮层神经元中,TG4-155(1 μM)可抑制PGE2诱导的cAMP积累,阻断EP2介导的CREB磷酸化,并降低癫痫相关促炎细胞因子(IL-1β、TNF-α)的mRNA表达[3] 2. 在SH-SY5Y神经母细胞瘤细胞中,TG4-155可浓度依赖性(0.1–10 μM)抑制PGE2诱导的cAMP生成,IC50为1.2 μM[3] 3. 在人结肠癌细胞系HT-29和乳腺癌细胞系MCF-7中,TG4-155(0.1–10 μM)可剂量依赖性抑制PGE2诱导的细胞增殖,IC50分别为0.8 μM和1.1 μM;同时还能抑制PGE2介导的基质金属蛋白酶MMP-9和MMP-2的表达,降低Transwell实验中的细胞侵袭能力[1] 4. TG4-155在体外对EP2受体具有高度选择性,对其他前列腺素受体的抑制活性微弱(IC50>10 μM),且不干扰PGE2与非EP2受体的结合[2] 5. 在人单核细胞来源的巨噬细胞(THP-1细胞)中,TG4-155(0.1–10 μM)可浓度依赖性降低LPS诱导的IL-6和PGE2分泌,并下调COX-2的mRNA表达[2] |
| 体内研究 (In Vivo) |
给予 TG4-155(5 mg/kg,腹腔注射;1 小时和 12 小时)可显着降低 C57BL/6 小鼠癫痫持续状态 (SE) 诱导的神经变性评分[3]。 TG4-155(3 mg/kg;腹腔注射)在 C57BL/ 中显示出 61% 的生物利用度(腹腔注射途径与静脉注射相比),血浆半衰期 (t1/2) 为 0.6 小时,脑/血浆比率为 0.3 6只小鼠[3]。动物模型:C57BL/6 小鼠(8-12 周龄)[3] 剂量:5 mg/kg 给药方式:腹腔注射; 1 小时和 12 小时结果:给药显着降低了海马 CA1 亚区中 SE 诱导的神经变性评分 91%,CA3 亚区降低了 80%,门门降低了 63%。动物模型:C57BL/6 小鼠[3] 剂量:3 mg/kg 给药方式:腹腔注射 结果:显示生物利用度为 61%(腹腔注射途径与静脉注射相比),t1/2 为 0.6 小时,脑/血浆比率为 0.3 。
1. 在大鼠匹罗卡品诱导的癫痫持续状态模型中,腹腔注射TG4-155(30 mg/kg,每日两次,连续7天)可显著减少海马区神经元的凋亡和坏死,减轻癫痫发作后的神经元损伤,降低脑内IL-1β和TNF-α的蛋白水平;同时还能减少癫痫发作的频率和持续时间,改善神经功能缺损评分[3] 2. 在裸鼠HT-29结肠癌异种移植模型中,口服TG4-155(10 mg/kg,每日一次,连续21天)可抑制约60%的肿瘤生长,下调肿瘤组织中MMP-9和VEGF的表达,减少微血管密度[1] 3. 在小鼠乳腺癌肺转移模型中,腹腔注射TG4-155(20 mg/kg,每日两次,连续14天)可使肺转移结节减少约70%[1] 4. 在小鼠角叉菜胶诱导的足肿胀模型中,造模前30分钟腹腔注射TG4-155(5 mg/kg),可显著降低造模后1、3、6小时的足肿胀程度,减少足跖组织中PGE2和IL-6的含量[2] 5. 接受治疗剂量TG4-155的癫痫模型大鼠,其脑内未观察到明显的神经元损伤或炎症反应加重的现象[3] |
| 酶活实验 |
1. EP2受体结合实验流程:将重组人EP2受体膜蛋白与不同浓度的TG4-155及3H-PGE2共同孵育;孵育结束后,通过玻璃纤维滤膜分离结合态与游离态配体,用液体闪烁计数仪检测结合部分的放射性强度,以此计算TG4-155与EP2受体结合的Ki值[3]
2. cAMP积累实验流程:将表达人EP2的HEK293细胞用TG4-155预处理15分钟,再加入PGE2刺激30分钟;裂解细胞后,采用酶联免疫试剂盒检测cAMP浓度,绘制剂量-反应曲线并确定IC50[3] 3. 钙流实验流程:将稳定表达EP2的CHO细胞接种于96孔板,负载钙敏感荧光染料后与TG4-155孵育20分钟;加入PGE2刺激钙信号,用荧光酶标仪实时检测细胞内钙浓度变化,评估TG4-155对EP2介导钙流的抑制作用[2] 4. 前列腺素受体选择性实验流程:将其他前列腺素受体(EP1、EP3、EP4、DP1等)的膜蛋白与TG4-155及受体特异性放射性配体共同孵育,检测结合率以评估TG4-155的受体选择性[2] |
| 细胞实验 |
细胞系:PC3细胞
浓度:48小时 孵育时间:0.01、0.1和1 μM 结果:显着抑制PGE2诱导的癌细胞增殖,浓度为-依赖方式。 1. 癌细胞增殖实验流程:将HT-29和MCF-7细胞接种于96孔板,贴壁后用不同浓度的TG4-155与1 μM PGE2共同处理72小时;加入CCK-8试剂孵育2小时,用酶标仪检测450 nm处的吸光度,计算细胞增殖抑制率并确定IC50[1] 2. 细胞侵袭实验流程:在Transwell小室上室铺基质胶,下室加入含10% FBS的培养基;将经TG4-155处理的细胞悬液加入上室,培养24小时后,用结晶紫染色下室的侵袭细胞并计数,计算侵袭率[1] 3. 原代皮层神经元损伤实验流程:分离新生大鼠皮层神经元并接种于24孔板,培养7天后用TG4-155预处理1小时,再加入PGE2和谷氨酸刺激;24小时后用LDH试剂盒检测细胞损伤程度,Hoechst染色检测凋亡细胞比例;提取总RNA通过qPCR检测IL-1β和TNF-α的mRNA表达,提取总蛋白通过western blot检测CREB和p-CREB的表达[3] 4. 炎症因子表达实验流程:将THP-1细胞接种于6孔板并分化为巨噬细胞,用LPS刺激并加入TG4-155培养24小时;收集细胞上清,用ELISA检测IL-6和PGE2的浓度,提取总RNA通过RT-PCR检测COX-2的mRNA表达[2] |
| 动物实验 |
C57BL/6 mice (8-12 wk old)
5 mg/kg I.p.; at 1 and 12 h 1. For the rat epilepsy model: Adult Sprague-Dawley rats were intraperitoneally injected with pilocarpine (300 mg/kg) to induce status epilepticus; successfully modeled rats were randomly divided into vehicle and TG4-155 treatment groups; TG4-155 was dissolved in a mixture of 5% DMSO and 95% normal saline, and administered intraperitoneally at 30 mg/kg twice daily for 7 days; the vehicle group received an equal volume of solvent; seizure frequency and duration were recorded during the experiment; on day 7, rats were sacrificed, and hippocampal tissues were collected for TUNEL and Nissl staining to evaluate neuronal damage, and ELISA was used to detect brain inflammatory factor levels [3] 2. For the nude mouse colon cancer xenograft model: HT-29 cells were subcutaneously inoculated into the right flank of Balb/c nude mice; when tumor volume reached approximately 100 mm³, mice were randomly divided into vehicle and TG4-155 treatment groups; TG4-155 was dissolved in 0.5% CMC-Na solution and administered by oral gavage at 10 mg/kg once daily for 21 days; tumor length and width were measured twice weekly to calculate tumor volume; at the end of the experiment, mice were sacrificed, and tumor tissues were collected for immunohistochemical detection of MMP-9 and VEGF expression, and microvessel density was counted [1] 3. For the mouse carrageenan-induced paw edema model: ICR mice were used to establish an inflammation model by subcutaneous injection of 1% carrageenan solution into the right hind paw; TG4-155 (dissolved in normal saline containing 1% Tween 80) was intraperitoneally administered at 5 mg/kg 30 minutes before modeling; paw thickness was measured at 1, 3, and 6 hours post-modeling to calculate swelling rate; at the end of the experiment, paw tissues were collected for ELISA detection of PGE2 and IL-6 concentrations [2] 4. For the mouse breast cancer lung metastasis model: MCF-7 cells were injected into the tail vein of Balb/c nude mice to establish a lung metastasis model; TG4-155 was dissolved in normal saline containing 1% Tween 80 and administered intraperitoneally at 20 mg/kg twice daily for 14 days; at the end of the experiment, mice were sacrificed, and lung tissues were dissected to count metastatic nodules [1] |
| 药代性质 (ADME/PK) |
1. TG4-155 has a good oral bioavailability of approximately 45% in rats; after oral administration of 10 mg/kg, the maximum plasma concentration (Cmax) is 1.2 μM, the time to reach Cmax (Tmax) is 1.5 hours, and the plasma half-life (t1/2) is 3.2 hours [2]
2. In mice, TG4-155 is mainly distributed in the brain, liver, and spleen, with brain concentration reaching 30% of plasma concentration; it can rapidly cross the blood-brain barrier in rats, and brain concentration peaks at 1 hour after intraperitoneal injection, accounting for 25% of plasma concentration [2][3] 3. TG4-155 is mainly metabolized in the liver by CYP3A4-catalyzed oxidation; metabolites are excreted through urine and feces, with an excretion rate of approximately 70% within 24 hours [2] |
| 毒性/毒理 (Toxicokinetics/TK) |
1. In acute toxicity studies, TG4-155 showed no obvious lethality in rats at an oral dose of up to 200 mg/kg, and the LD50 in mice was >150 mg/kg via intraperitoneal injection [2]
2. In a 28-day subchronic toxicity study in rats, oral administration of TG4-155 at 10, 30, and 100 mg/kg/day caused mild hepatic steatosis only at the 100 mg/kg dose, which was reversible after drug withdrawal; no renal toxicity or hematological toxicity was observed [2] 3. The plasma protein binding rate of TG4-155 is approximately 85% in humans, rats, and mice [2] 4. Co-administration of TG4-155 with the CYP3A4 inhibitor ketoconazole increased the plasma AUC of TG4-155 by approximately 2-fold, indicating potential drug-drug interactions [2] 5. In epilepsy model rats, continuous administration of TG4-155 at a therapeutic dose (30 mg/kg) for 7 days caused no obvious behavioral abnormalities, weight loss, or histopathological changes in the liver and kidney [3] |
| 参考文献 |
|
| 其他信息 |
1. TG4-155 is the first small-molecule selective EP2 receptor antagonist developed by TG Therapeutics, initially designed for cancer treatment due to the high expression of EP2 receptor in various tumors (colon, breast, lung) and its role in mediating tumor proliferation, invasion, and angiogenesis [1]
2. TG4-155 exerts neuroprotective effects in epilepsy models by blocking the PGE2-EP2 pathway-mediated inflammatory response and neuronal apoptosis, and is a potential therapeutic agent for post-epileptic neuronal damage [3] 3. The EP2 receptor is a key receptor for PGE2, involved in multiple pathological processes such as inflammation, cancer, and neurodegenerative diseases; TG4-155 as a selective EP2 antagonist has potential for multi-indication development and is currently in preclinical research with no clinical trials or FDA warning information [2] |
| 分子式 |
C23H26N2O4
|
|---|---|
| 分子量 |
394.463546276093
|
| 精确质量 |
394.189
|
| 元素分析 |
C, 70.03; H, 6.64; N, 7.10; O, 16.22
|
| CAS号 |
1164462-05-8
|
| PubChem CID |
5886965
|
| 外观&性状 |
Light yellow to yellow solid powder
|
| LogP |
4.196
|
| tPSA |
61.72
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
29
|
| 分子复杂度/Complexity |
541
|
| 定义原子立体中心数目 |
0
|
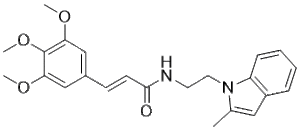
| SMILES |
O=C(/C=C/C1C=C(C(=C(C=1)OC)OC)OC)NCCN1C(C)=CC2C=CC=CC1=2
|
| InChi Key |
YBHUXHFZLMFETJ-MDZDMXLPSA-N
|
| InChi Code |
InChI=1S/C23H26N2O4/c1-16-13-18-7-5-6-8-19(18)25(16)12-11-24-22(26)10-9-17-14-20(27-2)23(29-4)21(15-17)28-3/h5-10,13-15H,11-12H2,1-4H3,(H,24,26)/b10-9+
|
| 化学名 |
(E)-N-[2-(2-methylindol-1-yl)ethyl]-3-(3,4,5-trimethoxyphenyl)prop-2-enamide
|
| 别名 |
TG4155; TG4-155; TG-4-155; TG-4155; TG 4-155; TG 4155
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: 79~125 mg/mL (200.3~316.9 mM)
Ethanol: ~5 mg/mL |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (5.27 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (5.27 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL 澄清 DMSO 储备液添加到 900 μL 玉米油中并混合均匀。 View More
配方 3 中的溶解度: 5%DMSO + Corn oil: 4.0mg/ml (10.14mM) 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5351 mL | 12.6756 mL | 25.3511 mL | |
| 5 mM | 0.5070 mL | 2.5351 mL | 5.0702 mL | |
| 10 mM | 0.2535 mL | 1.2676 mL | 2.5351 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
|