| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 500mg |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
在 A549 细胞中,百里酚(20 μM,24-48 小时)影响 IL4I1(24 小时)和芳香通道受体 (AHR) 信号通路(48 小时)的表达 [2]。百里酚(5 μM,48 小时) A549 百里酚(0-600 μM,48 小时)调节可抑制多种二元类型(Cal27、SCC4、SCC9、HeLa、H460、 MDA-231 和 PC3 细胞)[2]。在 A 中,百里酚 (200 μg/mL) 会产生依赖于半胱天冬酶的分生孢子丙酮。黄疸[4]。
|
|---|---|
| 体内研究 (In Vivo) |
在原位小鼠肺腺癌 (LUAD) 模型中,单独使用百里香酚(75 mg/kg,腹腔注射)以及与抗 PD-1 抗体(10 mg/kg,腹腔注射)联合使用均可降低 LUAD 进展并使动物更容易受到抗 PD-1 抗体的影响。 -PD-1拮抗剂治疗[2]。在 Cal27 衍生的异种移植小鼠中,百里香酚(4.3 mM,溶于 50 μL 无菌盐水,瘤内注射,每日)显示出抗癌功效。
|
| 细胞实验 |
蛋白质印迹分析 [2]
细胞类型: A549 细胞 测试浓度: 20 μM 孵育。 孵育时间: 48 小时 实验结果: IL4I1 蛋白水平和 AHR 核易位的抑制。总体 AHR 水平降低。 |
| 动物实验 |
Animal/Disease Models: Orthotopic mouse LUAD model [2]
Doses: Thymol 75 mg/kg, anti-PD-1 antibody 10 mg/kg Route of Administration: anti-PD-1 antibody: intraperitoneal (ip) injection, once every 1 week. Day 5 after tumor injection. Thymol: intraperitoneally (ip) (ip), every 2 days starting on day 5 after tumor injection. Experimental Results: Inhibited the progression of LUAD and improved the survival rate of mice. Reduce IL4I1 levels in tumors. Improve the efficacy of anti-PD-1 antibodies. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Aromatic herbs as feed additives in animal production are encountering growing interest, but data on the fate of the aromatic compounds from the plant in the animal body are very scarce. In the present study, thyme (Thymus vulgaris) herb consisting of leaves and flowers without stems was used as an ingredient in the diet for broilers. The herb was fed for 35 days to five groups of broilers (0, 0.1, 0.2, 0.3, and 1% w/w in the diet). Animal performance and the concentrations of the main essential oil component from thyme, thymol, were measured in gut contents, plasma and liver and muscle tissues using solid phase microextraction and gas chromatography/mass spectrometry. There were no differences between the groups in feed intake, daily weight gain, feed conversion and slaughter weight. Thymol was detected in gut contents, plasma and liver and muscle tissues. Increased intestinal thymol concentrations were found in the group with 1% thyme compared with the other groups (P<0.05). In liver and muscle tissues the thymol levels were close to the limit of quantification. The data do not indicate a positive effect of thyme on animal performance. With high dietary levels of thyme herb, thymol concentrations increased in gut contents and plasma but were very low in edible tissues such as liver and flesh. Thymol is readily absorbed from the gastrointestinal tract following oral administration. It is essentially excreted in the urine within the first 24 hours after absorption. Metabolism / Metabolites Only small amounts of the absorbed substance undergo urinary excretion as hydroxylated compounds. Thymol is predominantly excreted unchanged and in the form of its glucuronide and sulfate conjugates. Substituted monophenols, thymol ... which occur in essential oils of plants, particularly thyme, are ... conjugated with glucuronic acid & sulfate. Thymol has known human metabolites that include p-Cymen-8-en-3-ol, p-Cymene-2,3-diol, thymol sulfate, thymol O-glucuronide, and Thymoquinol. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Thymol forms colorless crystals, often large, or white crystalline powder. It is used as a pesticide (insecticide, fungicide, rodenticide, antimicrobial); also used in perfumery; as a mold and mildew preventive; in microscopy; as a preservative; antioxidant; flavoring; and lab reagent. Thymol is included in an FDA over-the-counter drug used as an antibacterial and antifungal agent, and approved as an excipient. HUMAN EXPOSURE AND TOXICITY: In humans, thymol on its own or as an ingredient in combination preparations, considering its wide use, has led to primary skin irritation and skin sensitisation only in rare cases. Thymol is a mild local irritant. It resembles phenol in its systemic actions but is less toxic, partly because it is less soluble. It produces gastric pain, nausea, vomiting, central hyperactivity (eg, talkativeness), occasionally convulsions, coma, cardiac and respiratory collapse. Thymol was not genotoxic in the human colon carcinoma cell line Caco-2. ANIMAL STUDIES: On acute oral administration, thymol is harmful whereas it is practically non-toxic following acute dermal application. In the rabbit, thymol is corrosive to the skin and eye. Rats subjected to subchronic administration in the feed for a period of 19 weeks tolerate thymol at 10000 ppm. Thymol did not increase the incidence of spontaneous lung tumors in mice. In embryonic chickens, thymol causes multiple malformations on injection into the air bubble or the yolk sac. In vivo, oral administration of thymol does not induce micronuclei in mice even in the toxic dose range. In the Salmonella/microsome assay, thymol exhibits no mutagenic effect; however, it has been reported to give positive results in the UDS test (liquid scintillation) and in the SCE test with embryonic cells of the Syrian hamster. The findings are statistically significant, though there is no strict dose-response relationship. The various other actions of thymol include cytotoxic, antineoplastic, antibacterial, fungicidal, anti-inflammatory, spasmolytic and other pharmacodynamic effects. ECOTOXICITY STUDIES: The effects of thymol on olfactory memory and gene expression in the brain of the honeybee were explored in bees previously exposed to thymol, and the specificity of the bee response to the conditioned stimulus was lost 24 hr after learning. The results also indicated that the genes coding for the cellular targets of thymol could be rapidly regulated after exposure to this molecule. Essential oils (including thymol) are used by beekeepers to control the Varroa mites infesting honeybee colonies. Interactions Thymol (TOH) was investigated for its ability to protect against mercuric chloride (HgCl2 )-induced cytotoxicity and genotoxicity using human hepatocarcinoma (HepG2) cell line. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay confirmed the efficacy of TOH pretreatment in attenuating HgCl2 -induced cytotoxicity. Pretreatment with TOH inhibited HgCl2 -induced genotoxicity, depolarization of mitochondrial membrane, oxidative stress, and mitochondrial superoxide levels. Interestingly, TOH (100 uM) alone elevated the intracellular basal glutathione S-transferase (GST) levels and TOH pretreatment abrogated the decrease in glutathione, GST, superoxide dismutase, and catalase levels even after HgCl2 intoxication. Furthermore, TOH was also capable of inhibiting HgCl2 -induced apoptotic as well as necrotic cell death analyzed by flowcytometric analysis of cells dual stained with Annexin-FITC/propidium iodide. The present findings clearly indicate the cytoprotective potential of TOH against HgCl2 -induced toxicity, which may be attributed to its free radical scavenging ability which facilitated in reducing oxidative stress and mitochondrial damage thereby inhibiting cell death. Thymol is a natural monoterpene phenol primarily found in thyme, oregano, and tangerine peel. It has been shown to possess anti-inflammatory property both in vivo and in vitro. /The present paper studied/ the anti-inflammatory effect of thymol in lipopolysaccharide (LPS)-stimulated mouse mammary epithelial cells (mMECs). The mMECs were stimulated with LPS in the presence or absence of thymol (10, 20, 40 ug/mL). The concentrations of tumor necrosis factor a (TNF-a), interleukin (IL)-6, and IL-1beta in the supernatants of culture were determined using enzyme-linked immunosorbent assay. Cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), extracellular signal-regulated protein kinase (ERK), c-Jun N-terminal kinase (JNK), nuclear factor-kappaB (NF-kappaB), and inhibitor protein of NF-kappaB (IkappaBa) were measured using western blot. The results showed that thymol markedly inhibited the production of TNF-a and IL-6 in LPS-stimulated mMECs. The expression of iNOS and COX-2 was also suppressed by thymol in a dose-dependent manner. Furthermore, thymol blocked the phosphorylation of IkappaBa, NF-kappaB p65, ERK, JNK, and p38 mitogen-activated protein kinases (MAPKs) in LPS-stimulated mMECs. These results indicate that thymol exerted anti-inflammatory property in LPS-stimulated mMECs by interfering the activation of NF-kappaB and MAPK signaling pathways. Thereby, thymol may be a potential therapeutic agent against mastitis. Non-Human Toxicity Values LD50 Rat oral 980 mg/kg LD50 Mouse oral 640 mg/kg LD50 Mouse iv 100 mg/kg LD50 Rat dermal >2000 mg/kg |
| 参考文献 |
|
| 其他信息 |
Thymol is a phenol that is a natural monoterpene derivative of cymene. It has a role as a volatile oil component. It is a member of phenols and a monoterpenoid. It derives from a hydride of a p-cymene.
A phenol obtained from thyme oil or other volatile oils. It is used as a stabilizer in pharmaceutic preparations. It has been used for its antiseptic, antibacterial, and antifungal actions, and was formerly used as a vermifuge. (Dorland, 28th ed) Thymol has been reported in Acanthospermum australe, Humulus lupulus, and other organisms with data available. A phenol obtained from thyme oil or other volatile oils used as a stabilizer in pharmaceutical preparations, and as an antiseptic (antibacterial or antifungal) agent. See also: Paeonia lactiflora root (part of); Elymus repens root (part of); Eucalyptol; thymol (component of) ... View More ... Mechanism of Action The potent role of thymol, a natural compound, in modulation of macrophage activity was evaluated by determining all the sequential steps involved during phagocytosis. We found a significant increase in the proliferation of splenocytes in the presence of thymol and it proved to be a good mitogen. Uptake capacity of macrophages was enhanced due to increased membrane fluidity after treatment with thymol and it also increases lysosomal activity of macrophages. Data of superoxide anion generation revealed the involvement of thymol in the generation of respiratory burst as it potentiated this property of macrophages at a concentration of 150 uM. In the case of TNF-a, IL-1beta and PGE(2) a decreased level of secretion was observed 154 pg/mL, 736.1 pg/mL, and 151 pg/mL respectively when compared with lipopolysaccharide treated cells, where the level of these cytokines was significantly high. We also determined the anti-complementary activity of thymol which showed to be more effective than rosmarinic acid. Thus, the results obtained from the study suggest the potential role of thymol as a natural immunostimulatory drug which can be used in the treatment of various immunological disorders. Therapeutic Uses Anti-Infective Agents; Anti-Infective Agents, Local; Antifungal Agents EXPL THER Thymol, a naturally occurring monocyclic phenolic compound derived from Thymus vulgaris (Lamiaceae), has been reported to exhibit anti-inflammatory property in vivo and vitro. However, the mechanism of thymol is not clear. The aim of the present study was to investigate the effects of thymol on allergic inflammation in OVA-induced mice asthma and explore its mechanism. The model of mouse asthma was established by the induction of OVA. Thymol was orally administered at a dose of 4, 8, and 16 mg/kg body weight 1hr before OVA challenge. At 24h after the last challenge, mice were sacrificed, and the data were collected by various experimental methods. The results revealed that pretreatment with thymol reduced the level of OVA-specific IgE, inhibited recruitment of inflammatory cells into airway, and decreased the levels of IL-4, IL-5, and IL-13 in BALF. Moreover, the pathologic changes of lung tissues were obviously ameliorated and goblet cell hyperplasia was effectively inhibited by the pretreatment of thymol. In addition, thymol reduced the development of airway hyperresponsiveness and blocked the activation of NF-kappaB pathway. All data suggested that thymol ameliorated airway inflammation in OVA-induced mouse asthma, possibly through inhibiting NF-kappaB activation. These findings indicated that thymol may be used as an alternative agent for treating allergic asthma. EXPL THER Obesity has become a worldwide health problem. Most of the synthetic anti-obesity drugs have failed to manage the obesity due to either ineffectiveness or adverse effect. The research of prominent chemical constituents from herbal for the management of obesity has greatly increased. The main objective of the present study was intended to examine the effects of thymol in high-fat diet (HFD)-induced obesity in murine model. Male Wistar rats were fed HFD for 6 weeks to induce obesity. Thymol (14 mg/kg) administered orally twice a day to HFD-fed rats for 4 weeks. Alteration in body weight gain, visceral fat-pads weight and serum biochemical markers were assessed. At the end of study, rats fed with HFD exhibited significantly (p< 0.001) enhanced body weight gain, visceral pad weight, lipids, alanine aminotransferase (ALT), aspartate aminotransaminase (AST), lactate dehydrogenase (LDH), blood urea nitrogen (BUN), glucose, insulin and leptin levels compared with rats fed with normal diets. Thymol treatment showed significantly (p< 0.001) decreased body weight gain, visceral fat-pad weights, lipids, ALT, AST, LDH, BUN, glucose, insulin, and leptin levels in HFD-induced obese rats. Furthermore, thymol treatment showed significantly decreased serum lipid peroxidation and increased antioxidant levels in HFD-induced obese rats. Thymol prevents HFD-induced obesity in murine model through several mechanisms including attenuation of visceral fat accumulation, lipid lowering action, improvement of insulin and leptin sensitivity and enhanced antioxidant potential. EXPL THER Mast cells play a critical role in inflammatory skin diseases through releasing proinflammatory mediators; however, few therapies directly target these cells. In 1878, the use of topical thymol, a now recognized potent agonist for transient receptor potential channels, was first described to treat eczema and psoriasis. /The objective was/ to determine the mechanisms through which thymol can alter skin inflammation. METHODS: /This study/ examined the effect of topical thymol on IgE-dependent responses using a mast cell-dependent passive cutaneous anaphylaxis (PCA) model, as well as in vitro-cultured mast cells. Thymol dose-dependently inhibited PCA when administered topically 24 hours before antigen challenge but provoked an ear-swelling response directly on application. This direct effect was associated with local mast cell degranulation and was absent in histamine-deficient mice. However, unlike with PCA responses, there was no late-phase swelling. In vitro thymol directly triggered calcium flux in mast cells through transient receptor potential channel activation, along with degranulation and cytokine transcription. However, no cytokine protein was produced. Instead, thymol induced a significant increase in apoptotic cell death that was seen both in vitro and in vivo. /The authors/ propose that the efficacy of thymol in reducing IgE-dependent responses is through promotion of activation-induced apoptotic cell death of mast cells and that this likely explains the clinical benefits observed in early clinical reports. For more Therapeutic Uses (Complete) data for THYMOL (8 total), please visit the HSDB record page. |
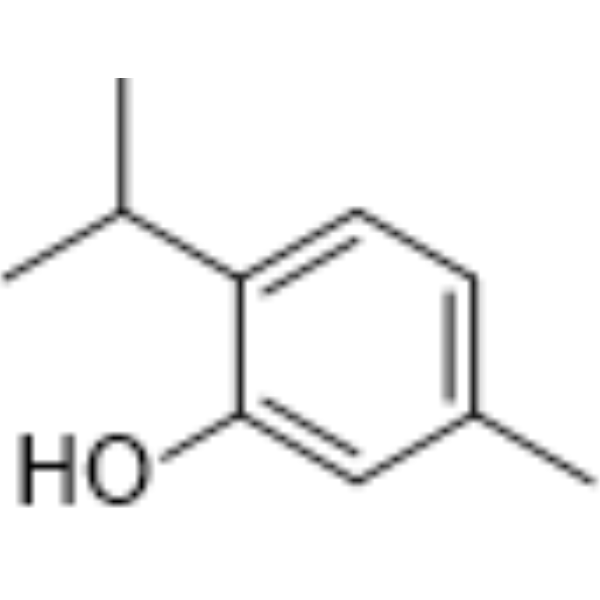
| 分子式 |
C10H14O
|
|---|---|
| 分子量 |
150.22
|
| 精确质量 |
150.104
|
| CAS号 |
89-83-8
|
| 相关CAS号 |
Thymol-d13;1219798-93-2
|
| PubChem CID |
6989
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.0±0.1 g/cm3
|
| 沸点 |
233.0±0.0 °C at 760 mmHg
|
| 熔点 |
48-51 °C(lit.)
|
| 闪点 |
102.2±0.0 °C
|
| 蒸汽压 |
0.0±0.4 mmHg at 25°C
|
| 折射率 |
1.523
|
| LogP |
3.28
|
| tPSA |
20.23
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
1
|
| 可旋转键数目(RBC) |
1
|
| 重原子数目 |
11
|
| 分子复杂度/Complexity |
120
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
MGSRCZKZVOBKFT-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C10H14O/c1-7(2)9-5-4-8(3)6-10(9)11/h4-7,11H,1-3H3
|
| 化学名 |
5-methyl-2-propan-2-ylphenol
|
| 别名 |
NSC-11215; NSC 11215; Thymol
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~125 mg/mL (~832.11 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (13.85 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (13.85 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (13.85 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 6.6569 mL | 33.2845 mL | 66.5690 mL | |
| 5 mM | 1.3314 mL | 6.6569 mL | 13.3138 mL | |
| 10 mM | 0.6657 mL | 3.3285 mL | 6.6569 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。