| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| Other Sizes |
|
| 靶点 |
|
|
|---|---|---|
| 体外研究 (In Vitro) |
Tipranavir (PNU-140690) 对多种野生型和多重 PI 耐药的 HIV-1 变体表现出强大的作用,抑制 HIV-1 蛋白酶的酶活性,并防止蛋白酶亚基的二聚化。 HIV11MIX 是 11 种多重 PI 耐药(但 TPV 敏感)临床分离株(包括 HIVB 和 HIVC)的混合物,可快速产生高水平替拉那韦 (PNU-140690) 耐药性,并在高浓度替拉那韦 (PNU-140690) 下复制在针对替拉那韦进行选择后(通过 10 代 [HIV11MIXP10])。 cHIVBI54V 和 cHIVBI54V/V82T 对替拉那韦 (PNU-140690) 有相当大的耐药性,其 IC50 分别为 2.9 和 3.2 μM,与针对 cHIVB 的 IC50 相比增加了 11 倍和 12 倍。
|
|
| 体内研究 (In Vivo) |
为了提高地普拉那韦 (PNU-140690) 的生物利用度,有必要将其与低剂量利托那韦 (RTV) 联合使用,每天口服两次。替拉那韦/r 联合治疗小鼠的肝脏、脾脏和眼睛中替拉那韦 (PNU-140690) 的丰度明显高于替拉那韦治疗的动物。在单独使用替拉那韦组中,血清和肝脏中分别有 31% 和 38% 由替拉那韦代谢物 (PNU-140690) 组成。在分别用替拉那韦 (PNU-140690) 和替拉那韦 (TPV/r) 联合治疗的小鼠的血清和肝脏中,仅发现 1% 和 2% 的代谢物。 Sprague-Dawley 大鼠与 RTV 联合给予一剂[14C]Tipranavir (PNU-140690)。粪便中含有大量与氧化相关的代谢物。没有发现任何一种代谢物大量存在于尿液中[2]。
|
|
| 动物实验 |
|
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Absorption is limited, although no absolute quantification of absorption is available. Tipranavir is extensively bound to plasma proteins (>99.9%). It binds to both human serum albumin and a-1-acid glycoprotein. The mean fraction of tipranavir (dosed without ritonavir) unbound in plasma was similar in clinical samples from healthy volunteers and HIV-1 positive patients. Total plasma tipranavir concentrations for these samples ranged from 9 to 82 uM. The unbound fraction of tipranavir appeared to be independent of total drug concentration over this concentration range. Administration of (14)C-tipranavir to subjects (n=8) that received Aptivus/ritonavir 500/200 mg dosed to steady-state demonstrated that most radioactivity (median 82.3%) was excreted in feces, while only a median of 4.4% of the radioactive dose administered was recovered in urine. In addition, most radioactivity (56%) was excreted between 24 and 96 hours after dosing. The effective mean elimination half-life of tipranavir/ritonavir in healthy volunteers (n=67) and HIV-1 infected adult patients (n=120) was approximately 4.8 and 6.0 hours, respectively, at steady state following a dose of 500/200 mg twice daily with a light meal. The pharmacokinetic and metabolite profiles of the antiretroviral agent tipranavir (TPV), administered with ritonavir (RTV), in nine healthy male volunteers were characterized. Subjects received 500-mg TPV capsules with 200-mg RTV capsules twice daily for 6 days. They then received a single oral dose of 551 mg of TPV containing 90 uCi of [(14)C]TPV with 200 mg of RTV on day 7, followed by twice-daily doses of unlabeled 500-mg TPV with 200 mg of RTV for up to 20 days. Blood, urine, and feces were collected for mass balance and metabolite profiling. Metabolite profiling and identification was performed using a flow scintillation analyzer in conjunction with liquid chromatography-tandem mass spectrometry. The median recovery of radioactivity was 87.1%, with 82.3% of the total recovered radioactivity excreted in the feces and less than 5% recovered from urine. Most radioactivity was excreted within 24 to 96 hr after the dose of ((14)C)TPV. Radioactivity in blood was associated primarily with plasma rather than red blood cells. Unchanged TPV accounted for 98.4 to 99.7% of plasma radioactivity. Similarly, the most common form of radioactivity excreted in feces was unchanged TPV, accounting for a mean of 79.9% of fecal radioactivity. The most abundant metabolite in feces was a hydroxyl metabolite, H-1, which accounted for 4.9% of fecal radioactivity. TPV glucuronide metabolite H-3 was the most abundant of the drug-related components in urine, corresponding to 11% of urine radioactivity. In conclusion, after the coadministration of TPV and RTV, unchanged TPV represented the primary form of circulating and excreted TPV and the primary extraction route was via the feces. The in vitro plasma protein binding of tipranavir was very high (> 99.9%) in all species including humans, with only a slight trend towards saturation over the concentration range of 10 to 100 um. Tipranavir with or without ritonavir co-administration, distributed primarily in the liver, small intestine, large intestine, kidney and lung. Tipranavir did not cross the blood-brain barrier and did not readily partitioning into red blood cells. Following intravenous dosing, tipranavir demonstrated low clearance ranging from 0.08 L/hr/kg in dogs to 1.15 l/h/kg in mice. The Vss ranged from 0.13 L/kg in dogs to 0.51 L/kg in rats. TPV was eliminated rapidly with a terminal t1/2 ranging from 0.93 hr in dogs to 5.43 hr in rats. Following oral dosing, tipranavir exhibited a mean Tmax ranging from 0.5 to 8 hr in all species. In all species a moderate or poor oral bioavailability of tipranavir was revealed, due to a lack of absorption and/or intestinal metabolism. Whereas the bioavailability in rats showed moderately levels of 28.0%, the bioavailability in dogs (6.5% and 7.7%) and also in mice (11%) and rabbits (9.9%) was minimal. Food had no significant effect on tipranavir oral bioavailability in dogs. Ritonavir co-administration studies were performed to investigate the benefit gained by the combination. However the use of different doses of ritonavir for oral and intravenous PK of tipranavir does not allow a clear comparison of tipranavir bioavailability with or without ritonavir. With ritonavir co-administration, following intravenous dosing, tipranavir demonstrated low to moderate clearance ranging from 0.0182 L/hr/kg in rats to 3.00 L/hr/kg in mice. In rats and dogs, co-administration of ritonavir resulted in a 4- to 5-fold decrease in clearance for tipranavir, which would be consistent with inhibition of drug-metabolising enzymes by ritonavir. Metabolism / Metabolites Hepatic. In vitro metabolism studies with human liver microsomes indicated that CYP 3A4 is the predominant CYP enzyme involved in tipranavir metabolism. Tipranavir (TPV) is the first nonpeptidic protease inhibitor used for the treatment of drug-resistant HIV infection. Clinically, TPV is coadministered with ritonavir (RTV) to boost blood concentrations and increase therapeutic efficacy. The mechanism of metabolism-mediated drug interactions associated with RTV-boosted TPV is not fully understood. In the current study, TPV metabolism was investigated in mice using a metabolomic approach. TPV and its metabolites were found in the feces of mice but not in the urine. Principal component analysis of the feces metabolome uncovered eight TPV metabolites, including three monohydroxylated, three desaturated, one dealkylated, and one dihydroxylated. In vitro study using human liver microsomes recapitulated five TPV metabolites, all of which were suppressed by RTV. CYP3A4 was identified as the primary enzyme contributing to the formation of four TPV metabolites (metabolites II, IV, V, and VI), including an unusual dealkylated product arising from carbon-carbon bond cleavage. Multiple cytochromes P450 (2C19, 2D6, and 3A4) contributed to the formation of a monohydroxylated metabolite (metabolite III). In vivo, RTV cotreatment significantly inhibited eight TPV metabolic pathways. In summary, metabolomic analysis revealed two known and six novel TPV metabolites in mice, all of which were suppressed by RTV. The current study provides solid evidence that the RTV-mediated boosting of TPV is due to the modulation of P450-dependent metabolism. The pharmacokinetic and metabolite profiles of the antiretroviral agent tipranavir (TPV), administered with ritonavir (RTV), in nine healthy male volunteers were characterized. Subjects received 500-mg TPV capsules with 200-mg RTV capsules twice daily for 6 days. They then received a single oral dose of 551 mg of TPV containing 90 uCi of [(14)C]TPV with 200 mg of RTV on day 7, followed by twice-daily doses of unlabeled 500-mg TPV with 200 mg of RTV for up to 20 days. ... The most abundant metabolite in feces was a hydroxyl metabolite, H-1, which accounted for 4.9% of fecal radioactivity. TPV glucuronide metabolite H-3 was the most abundant of the drug-related components in urine, corresponding to 11% of urine radioactivity. ... In vitro metabolism studies indicated that CYP3A4 is the predominant CYP isoform involved in tipranavir metabolism in humans. CYP3A isozyme was also identified in rat as the predominant CYP isoform involved in tipranavir metabolism. Studies in rats and humans dosed by tipranavir co-administered with ritonavir were conducted to assess metabolites. The unchanged tipranavir was the predominant form in plasma (>85.7%). Unchanged tipranavir was also the major form excreted in feces and urine. Combined levels of excreted metabolites in feces and urine accounted for approximately 4.8% and 7.4% in male and female rats. Only small amounts of a glucuronide were observed in faeces. For more Metabolism/Metabolites (Complete) data for Tipranavir (6 total), please visit the HSDB record page. Biological Half-Life 5-6 hours |
|
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Some degree of serum aminotransferase elevations occur in a high proportion of patients taking tipranavir containing antiretroviral regimens. Moderate-to-severe elevations in serum aminotransferase levels (>5 times the upper limit of normal) are found in 3% to 10% of patients, although rates may be higher in patients with HIV-HCV coinfection. These elevations are usually asymptomatic and self-limited and can resolve even with continuation of the medication. Clinically apparent liver injury from tipranavir is rare, and the clinical pattern of liver injury, latency and recovery have not been well defined. Several protease inhibitors have been associated with acute liver injury arising 1 to 8 weeks after onset, with variable patterns of liver enzyme elevation, from hepatocellular to cholestatic. Immunoallergic features (rash, fever, eosinophilia) are uncommon, as is autoantibody formation. The acute liver injury due to tipranavir is usually self-limited, but it can be severe, and isolated cases of acute liver failure have been reported to the sponsor. In HBV or HCV coinfected patients, some instances appear to be due to exacerbation of the underlying chronic liver disease, perhaps as a result of sudden immune reconstitution. Tipranavir therapy has not been clearly linked to lactic acidosis and acute fatty liver that is reported in association with several nucleoside analogue reverse transcriptase inhibitors. Thus, tipranavir is associated with a high rate of serum enzyme elevations which is generally higher than with other protease inhibitors, for which reason it is considered a second-line HIV protease inhibitor. Likelihood score: E* (unproven but suspected cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No published information is available on the use of tipranavir during breastfeeding. Tipranavir is not recommended during breastfeeding. Achieving and maintaining viral suppression with antiretroviral therapy decreases breastfeeding transmission risk to less than 1%, but not zero. Individuals with HIV who are on antiretroviral therapy with a sustained undetectable viral load and who choose to breastfeed should be supported in this decision. If a viral load is not suppressed, banked pasteurized donor milk or formula is recommended. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Gynecomastia has been reported among men receiving highly active antiretroviral therapy. Gynecomastia is unilateral initially, but progresses to bilateral in about half of cases. No alterations in serum prolactin were noted and spontaneous resolution usually occurred within one year, even with continuation of the regimen. Some case reports and in vitro studies have suggested that protease inhibitors might cause hyperprolactinemia and galactorrhea in some male patients, although this has been disputed. The relevance of these findings to nursing mothers is not known. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. Protein Binding Extensive (> 99.9%), to both human serum albumin and α-1-acid glycoprotein. Interactions Pharmacokinetic interaction with fluconazole (increased tipranavir concentrations; no change in fluconazole concentrations and AUC). If ritonavir-boosted tipranavir and fluconazole are used concomitantly, fluconazole dosage does not need to be adjusted but fluconazole dosage exceeding 200 mg daily is not recommended. If high fluconazole dosage is indicated, an alternative HIV PI or antiretroviral agent from another class should be considered. Possible pharmacokinetic interaction with carbamazepine, phenobarbital, or phenytoin (decreased tipranavir concentrations and possible decreased antiretroviral efficacy; altered carbamazepine concentrations). If used with carbamazepine or phenytoin, some experts suggest that anticonvulsant and tipranavir concentrations be monitored; alternatively, use of another anticonvulsant can be considered. Possible pharmacokinetic interaction with valproic acid (decreased plasma concentrations of valproic acid); possibility that the anticonvulsant may be less effective. Possible pharmacokinetic interaction with warfarin (altered warfarin concentrations). International normalized ratio (INR) should be monitored if warfarin is used concomitantly with ritonavir-boosted tipranavir, especially when initiating or discontinuing the antiretroviral agents; warfarin dosage should be adjusted as needed. Concomitant use of ritonavir-boosted tipranavir and an anticoagulant may increase the risk for bleeding;1 the drugs should be used concomitantly with caution. Possible pharmacokinetic interactions with amiodarone, bepridil (no longer commercially available in the US), flecainide, propafenone, or quinidine (increased plasma concentrations of the antiarrhythmic agent). Potential for serious and/or life-threatening adverse effects (e.g., cardiac arrhythmias). Concomitant use with ritonavir-boosted tipranavir is contraindicated. For more Interactions (Complete) data for Tipranavir (35 total), please visit the HSDB record page. |
|
| 参考文献 |
|
|
| 其他信息 |
Therapeutic Uses
Anti-HIV Agents Tipranavir with low-dose ritonavir (ritonavir-boosted tipranavir) is used in conjunction with other antiretroviral agents for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in adults, adolescents, and pediatric patients 2 years of age and older with evidence of viral replication who are antiretroviral-experienced and infected with HIV-1 strains resistant to multiple HIV protease inhibitors (PIs). /Included in US product labeling/ Drug Warnings /BOXED WARNING/ WARNING: HEPATOTOXICITY and INTRACRANIAL HEMORRHAGE. Hepatotoxicity: Clinical hepatitis and hepatic decompensation, including some fatalities, have been reported. Extra vigilance is warranted in patients with chronic hepatitis B or hepatitis C co-infection, as these patients have an increased risk of hepatotoxicity. Intracranial Hemorrhage: Both fatal and non-fatal intracranial hemorrhage have been reported. New onset diabetes mellitus, exacerbation of pre-existing diabetes mellitus and hyperglycemia have been reported during post-marketing surveillance in HIV-1 infected patients receiving protease inhibitor therapy. Some patients required either initiation or dose adjustments of insulin or oral hypoglycemic agents for treatment of these events. In some cases, diabetic ketoacidosis has occurred. In those patients who discontinued protease inhibitor therapy, hyperglycemia persisted in some cases. Because these events have been reported voluntarily during clinical practice, estimates of frequency cannot be made and a causal relationship between protease inhibitor therapy and these events has not been established. Aptivus should be used with caution in patients with a known sulfonamide allergy. Tipranavir contains a sulfonamide moiety. The potential for cross-sensitivity between drugs in the sulfonamide class and Aptivus is unknown. Rash, including maculopapular rash, urticarial rash, and possible photosensitivity reaction, has been reported in patients receiving ritonavir-boosted tipranavir. Rash occurred in 10% of women, 8% of men, and 21% of children receiving ritonavir-boosted tipranavir in clinical studies. The median time to onset of rash was 53 days and the median duration of rash was 22 days in adults. Rash accompanied by joint pain or stiffness, throat tightness, or generalized pruritus also has been reported. Discontinue tipranavir if severe rash develops. For more Drug Warnings (Complete) data for Tipranavir (16 total), please visit the HSDB record page. Pharmacodynamics Tipranavir is a non-peptidic protease inhibitor (PI) of HIV. Protease inhibitors block the part of HIV called protease. HIV-1 protease is an enzyme required for the proteolytic cleavage of the viral polyprotein precursors into the individual functional proteins found in infectious HIV-1. Nelfinavir binds to the protease active site and inhibits the activity of the enzyme. This inhibition prevents cleavage of the viral polyproteins resulting in the formation of immature non-infectious viral particles. Protease inhibitors are almost always used in combination with at least two other anti-HIV drugs. |
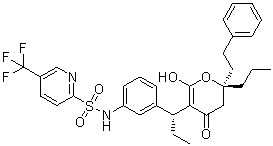
| 分子式 |
C31H33N2O5F3S
|
|---|---|
| 分子量 |
602.66432
|
| 精确质量 |
602.206
|
| CAS号 |
174484-41-4
|
| 相关CAS号 |
Tipranavir-d4;1217819-15-2
|
| PubChem CID |
54682461
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.313g/cm3
|
| 沸点 |
680ºC at 760mmHg
|
| 熔点 |
86-89ºC
|
| 闪点 |
365.1ºC
|
| 蒸汽压 |
0mmHg at 25°C
|
| 折射率 |
1.579
|
| LogP |
8.479
|
| tPSA |
113.97
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
10
|
| 可旋转键数目(RBC) |
11
|
| 重原子数目 |
42
|
| 分子复杂度/Complexity |
1050
|
| 定义原子立体中心数目 |
2
|
| SMILES |
CCC[C@]1(CC(=C(C(=O)O1)[C@H](CC)C2=CC(=CC=C2)NS(=O)(=O)C3=NC=C(C=C3)C(F)(F)F)O)CCC4=CC=CC=C4
|
| InChi Key |
SUJUHGSWHZTSEU-FYBSXPHGSA-N
|
| InChi Code |
InChI=1S/C31H33F3N2O5S/c1-3-16-30(17-15-21-9-6-5-7-10-21)19-26(37)28(29(38)41-30)25(4-2)22-11-8-12-24(18-22)36-42(39,40)27-14-13-23(20-35-27)31(32,33)34/h5-14,18,20,25,36-37H,3-4,15-17,19H2,1-2H3/t25-,30-/m1/s1
|
| 化学名 |
N-[3-[(1R)-1-[(2R)-4-hydroxy-6-oxo-2-(2-phenylethyl)-2-propyl-3H-pyran-5-yl]propyl]phenyl]-5-(trifluoromethyl)pyridine-2-sulfonamide
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~200 mg/mL (~331.86 mM)
Ethanol :≥ 50 mg/mL (~82.97 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 5 mg/mL (8.30 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
例如,若需制备1 mL的工作液,可将100 μL 50.0 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 5 mg/mL (8.30 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 50.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 View More
配方 3 中的溶解度: 2.5 mg/mL (4.15 mM) in 10% EtOH + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 配方 4 中的溶解度: 2.5 mg/mL (4.15 mM) in 10% EtOH + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL 澄清乙醇储备液加入到 900 μL 20% SBE-β-CD 生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 5 中的溶解度: ≥ 2.5 mg/mL (4.15 mM) (饱和度未知) in 10% EtOH + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL 澄清 EtOH 储备液加入900 μL 玉米油中,混合均匀。 配方 6 中的溶解度: 2.5 mg/mL (4.15 mM) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.6593 mL | 8.2966 mL | 16.5931 mL | |
| 5 mM | 0.3319 mL | 1.6593 mL | 3.3186 mL | |
| 10 mM | 0.1659 mL | 0.8297 mL | 1.6593 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Tipranavir Expanded Access Program (EAP) in PI-experienced Patients With HIV-1 Infection
CTID: NCT00097799
Phase: Status: Approved for marketing
Date: 2016-11-30