| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
Trandolapril(0.02 mM,1 mM;3 d)通过抑制细胞发育和诱导细胞凋亡来增加 K562 细胞系中凋亡细胞的比例 [2]。
|
|---|---|
| 体内研究 (In Vivo) |
通过限制肾间质基质表达和肌成纤维细胞活化,以及降低肾纤维化小鼠肾促炎细胞因子 RANTES 和 TNF-α 水平,曲诺普利(3 mg/kg/天;口服;7 天)可减少小鼠的阻塞性肾病 [2 ]。在大鼠中,Trundialolapril(0.3 mg/kg/天;口服;4 周)可减少细胞纤连蛋白积累、胶原蛋白和动脉肥大 [3]。在大鼠中,兰多普利的长期抗高血压作用(0.3 mg/kg/天;口服;4 个月)可降低血压 [3]。口服给药,每天两次,持续四个月,trundiapril (0.25 mg/kg) 可预防患有渡边遗传性高脂血症的兔子的动脉粥样硬化[4]。
|
| 细胞实验 |
细胞凋亡分析 [2]
细胞类型: K562、KU812、U937 和 HL60 测试浓度: 0-2 mM 孵育时间:0、1、2、3天 实验结果:1 mM抑制K562、KU812、U937,0.02 mM抑制HL60。 |
| 动物实验 |
Animal/Disease Models: UUD (unilateral ureteral obstruction) model [2] in male CD-1 mice (18-22 g)
Doses: 3 mg/kg Route of Administration: po (oral gavage); one time/day for 7 days Experimental Results: Caused Renal interstitial matrix expression, including fibronectin, type I and type III collagen, is diminished and, surprisingly, myofibroblast activation is inhibited through α-smooth muscle actin (a-SMA) expression, which reduces RANTES (regulated activation, normal T cell expression and secretion) and TNF-α levels. Animal/Disease Models: SHR model (spontaneous hypertensive rats, 4 weeks old) [3] Doses: 0.3 mg/kg Route of Administration: po (oral gavage); one time/day for 4 weeks Experimental Results: The collagen content in the aortic middle layer diminished, Arterial distensibility increases by approximately 80%. Animal/Disease Models: Watanabe hereditary hyperlipidemia rabbit (3 months old) [4] Doses: 0.25 mg/kg Route of Administration: po (oral gavage); twice a day; 9-month Experimental Results: Atherosclerosis on the intimal surface Sclerosis is diminished, and the cholesterol c |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
~ 40-60% absorbed; extensive first pass metabolism results in a low bioavailability of 4-14% After oral administration of trandolapril, about 33% of parent drug and metabolites are recovered in urine, mostly as trandolaprilat, with about 66% in feces. 18 L 52 L/h [After approximately 2 mg IV doses] /MILK/ Trandolapril and its metabolites are distributed into milk in rats. After oral administration of trandolapril, about 33% of parent drug and metabolites are recovered in urine, mostly as trandolaprilat, with about 66% in feces. The extent of the absorbed dose which is biliary excreted has not been determined. Plasma concentrations (Cmax and AUC of trandolapril and Cmax of trandolaprilat) are dose proportional over the 1-4 mg range, but the AUC of trandolaprilat is somewhat less than dose proportional. In addition to trandolaprilat, at least 7 other metabolites have been found, principally glucuronides or deesterification products. Serum protein binding of trandolapril is about 80%, and is independent of concentration. Binding of trandolaprilat is concentration-dependent, varying from 65% at 1000 ng/mL to 94% at 0.1 ng/mL, indicating saturation of binding with increasing concentration. Absolute bioavailability after oral administration of trandolapril is about 10% as trandolapril and 70% as trandolaprilat. After oral trandolapril under fasting conditions, peak trandolapril levels occur at about one hour and peak trandolaprilat levels occur between 4 and 10 hours. The elimination half-life of trandolapril is about 6 hours. At steady state, the effective half-life of trandolaprilat is 22.5 hours. Like all ACE inhibitors, trandolaprilat also has a prolonged terminal elimination phase, involving a small fraction of administered drug, probably representing binding to plasma and tissue ACE. During multiple dosing of trandolapril, there is no significant accumulation of trandolaprilat. Food slows absorption of trandolapril, but does not affect AUC or Cmax of trandolaprilat or Cmax of trandolapril. The volume of distribution of trandolapril is about 18 liters. Total plasma clearances of trandolapril and trandolaprilat after approximately 2 mg IV doses are about 52 liters/hour and 7 liters/hour respectively. Renal clearance of trandolaprilat varies from 1- 4 liters/hour, depending on dose. Metabolism / Metabolites Cleavage of the ester group of trandolapril, primarily in the liver, is responsible for conversion to trandolaprilat, the active metabolite. Seven other metabolites, including diketopiperazine and glucuronide conjugated derivatives of trandolapril and trandolaprilat, have been identified. Trandolapril is a prodrug and has little pharmacologic activity until hydrolyzed in the liver to trandolaprilat. Trandolapril's angiotensin-converting enzyme (ACE)-inhibiting activity is primarily due to its diacid metabolite, trandolaprilat. Cleavage of the ester group of trandolapril, primarily in the liver, is responsible for conversion. After oral administration of trandolapril, about 33% of parent drug and metabolites are recovered in urine, mostly as trandolaprilat, with about 66% in feces. ... In addition to trandolaprilat, at least 7 other metabolites have been found, principally glucuronides or deesterification products. Biological Half-Life The elimination half lives of trandolapril and trandolaprilat are about 6 and 10 hours, respectively, but, similar to all ACE inhibitors, trandolaprilat also has a prolonged terminal elimination phase that involves a small fraction of administered drug. This likely represents drug binding to plasma and tissue ACE. The effective half life of elimination for trandolaprilat is 16-24 hours. At steady state, the effective half-life of trandolaprilat is 22.5 hours. /Trandolaprilat/ |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Trandolapril is a colorless, crystalline solid. Trandolapril tablets are indicated for the treatment of hypertension. HUMAN STUDIES: In humans, the most likely clinical manifestation would be symptoms attributable to severe hypotension. Symptoms expected with angiotensin-converting enzyme (ACE) inhibitors are hypotension, hyperkalemia, and renal failure. Rare angiotensin-converting enzyme (ACE) inhibitor-associated clinical syndrome manifested initially by cholestatic jaundice may occur; this may progress to fulminant hepatic necrosis and is potentially fatal. Patients receiving an ACE inhibitor, including trandolapril, who develop jaundice or marked elevations in hepatic enzymes should discontinue the drug and receive appropriate monitoring. Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. ANIMAL STUDIES: In dogs, an oral dose of 1000 mg/kg did not cause mortality and abnormal clinical signs were not observed. In rats, an oral dose of 5000 mg/kg caused low mortality (1 male out of 5; 0 females). A perinatal and postnatal study was performed in female Sprague-Dawley rats treated orally with trandolapril at dosage levels of 3, 30 and 300 mg/kg/day from day 17 of pregnancy to postpartum day 21. Incidence of dilatation of renal pelvis with higher value in kidney weight was increased in offspring in the 30 and 300 mg/kg dosage groups, and water consumption at the same dosage groups was higher than that of the control group. Body weight gain in offspring was depressed in each treated group and viability of offspring from postpartum day 0 to day 4 was slightly decreased in the 30 and 300 mg/kg dosage groups comparing with that of the control group. No adverse effects were observed on the other postnatal development of the offspring, such as differentiation, sexual maturation, reflex, motor activity, emotionality, learning ability and reproductive performance. No adverse effects were detected in the second generation offspring. Hepatotoxicity Trandolapril, like other ACE inhibitors, has been associated with a low rate of serum aminotransferase elevations ( Likelihood score: E* (unproven but suspected rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Because no information is available on the use of trandolapril during breastfeeding, an alternate drug may be preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Serum protein binding of trandolapril is ~ 80% (independent of concentration and not saturable) while that of trandolaprilat is 65 to 94% (concentration-dependent and saturable). Interactions The hypotensive effect of certain inhalation anesthetics may be enhanced by angiotensin-converting enzyme (ACE) inhibitors including trandolapril. Patients taking concomitant mammalian target of rapamycin (mTOR)inhibitor (e.g., temsirolimus, sirolimus, everolimus) therapy may be at increased risk for angioedema. Nitritoid reactions (symptoms include facial flushing, nausea, vomiting and hypotension) have been reported rarely in patients on therapy with injectable gold (sodium aurothiomalate) and concomitant angiotensin-converting enzyme (ACE) inhibitor therapy including trandolapril. In patients who are elderly, volume-depleted (including those on diuretic therapy), or with compromised renal function, co- administration of non-steroidal anti-inflammatory drugs (NSAIDs), including selective cyclooxygenase-2 (COX-2) inhibitors, with angiotensin-converting enzyme (ACE) inhibitors, including trandolapril, may result in deterioration of renal function, including possible acute renal failure. These effects are usually reversible. Monitor renal function periodically in patients receiving trandolapril and NSAID therapy. The antihypertensive effect of ACE inhibitors, including trandolapril may be attenuated by NSAIDs. For more Interactions (Complete) data for Trandolapril (9 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Mouse (male) oral 4875 mg/kg LD50 Mouse (female) oral 3990 mg/kg |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Trandolapril is included in the database. Trandolapril tablets are indicated for the treatment of hypertension. It may be used alone or in combination with other antihypertensive medication such as hydrochlorothiazide. /Included in US product label/ Trandolapril tablets are indicated in stable patients who have evidence of left-ventricular systolic dysfunction (identified by wall motion abnormalities) or who are symptomatic from congestive heart failure within the first few days after sustaining acute myocardial infarction. Administration of trandolapril to Caucasian patients has been shown to decrease the risk of death (principally cardiovascular death) and to decrease the risk of heart failure-related hospitalization. /Included in US product label/ ACE inhibitors have been used in the management of heart failure, usually in conjunction with other agents such as cardiac glycosides, diuretics, and beta-blockers. /Angiotensin-converting enzyme (ACE) inhibitors; NOT included in US product label/ For more Therapeutic Uses (Complete) data for Trandolapril (6 total), please visit the HSDB record page. Drug Warnings /BOXED WARNING/ When pregnancy is detected, discontinue trandolapril as soon as possible. Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus. Angioedema of the face, extremities, lips, tongue, glottis, and larynx has been reported in patients treated with angiotensin-converting enzyme (ACE) inhibitors including trandolapril. Symptoms suggestive of angioedema or facial edema occurred in 0.13% of trandolapril-treated patients. Two of the four cases were life-threatening and resolved without treatment or with medication (corticosteroids). Angioedema associated with laryngeal edema can be fatal. If laryngeal stridor or angioedema of the face, tongue or glottis occurs, treatment with trandolapril should be discontinued immediately, the patient treated in accordance with accepted medical care and carefully observed until the swelling disappears. In instances where swelling is confined to the face and lips, the condition generally resolves without treatment; antihistamines may be useful in relieving symptoms. Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue trandolapril as soon as possible. These adverse outcomes are usually associated with use of these drugs in the second and third trimester of pregnancy. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. Appropriate management of maternal hypertension during pregnancy is important to optimize outcomes for both mother and fetus. /Angiotensin-converting enzyme (ACE) inhibitors/ Like other angiotensin-converting enzyme (ACE) inhibitors, trandolapril rarely is associated with hypotension in patients with uncomplicated hypertension. Symptomatic hypotension may occur; patients at particular risk include those with severe volume and/or salt depletion secondary to prolonged diuretic therapy, dietary salt restriction, dialysis, diarrhea, or vomiting. Volume and/or salt depletion should be corrected before starting trandolapril therapy. Marked hypotension, which may be associated with oliguria and/or progressive azotemia and rarely with acute renal failure and/or death, may occur in patients with heart failure (with or without associated renal impairment). In patients with heart failure, trandolapril therapy should be started at the recommended dose under close medical supervision with close monitoring for the first 2 weeks of treatment and whenever the dosage of trandolapril or diuretic is increased. Hypotension also should be avoided in patients with ischemic heart disease, aortic stenosis, or cerebrovascular disease. For more Drug Warnings (Complete) data for Trandolapril (13 total), please visit the HSDB record page. Pharmacodynamics Trandolapril is the ethyl ester prodrug of a nonsulfhydryl ACE inhibitor, trandolaprilat. Trandolapril is deesterified in the liver to the diacid metabolite, trandolaprilat, which is approximately eight times more active as an inhibitor of ACE than its parent compound. ACE is a peptidyl dipeptidase that is part of the RAAS. The RAAS is a homeostatic mechanism for regulating hemodynamics, water and electrolyte balance. During sympathetic stimulation or when renal blood pressure or blood flow is reduced, renin is released from the granular cells of the juxtaglomerular apparatus in the kidneys. In the blood stream, renin cleaves circulating angiotensinogen to ATI, which is subsequently cleaved to ATII by ACE. ATII increases blood pressure via a number of mechanisms. First, it stimulates the secretion of aldosterone from the adrenal cortex. Aldosterone travels to the distal convoluted tubule (DCT) and collecting tubule of nephrons where it increases sodium and water reabsorption by increasing the number of sodium channels and sodium-potassium ATPases on cell membranes. Second, ATII stimulates the secretion of vasopressin (also known as antidiuretic hormone or ADH) from the posterior pituitary gland. ADH stimulates further water reabsorption from the kidneys via insertion of aquaporin-2 channels on the apical surface of cells of the DCT and collecting tubules. Third, ATII increases blood pressure through direct arterial vasoconstriction. Stimulation of the Type 1 ATII receptor on vascular smooth muscle cells leads to a cascade of events resulting in myocyte contraction and vasoconstriction. In addition to these major effects, ATII induces the thirst response via stimulation of hypothalamic neurons. ACE inhibitors inhibit the rapid conversion of ATI to ATII and antagonize RAAS-induced increases in blood pressure. ACE (also known as kininase II) is also involved in the enzymatic deactivation of bradykinin, a vasodilator. Inhibiting the deactivation of bradykinin increases bradykinin levels and may further sustain the effects of trandolaprilat by causing increased vasodilation and decreased blood pressure. The blood pressure lowering effect of trandolaprilat is due to a decrease in peripheral vascular resistance, which is not accompanied by significant changes in urinary excretion of chloride or potassium or water or sodium retention. |
| 分子式 |
C24H34N2O5
|
|---|---|
| 分子量 |
430.54
|
| 精确质量 |
430.246
|
| CAS号 |
87679-37-6
|
| 相关CAS号 |
Trandolapril hydrochloride;87725-72-2;Trandolapril-d5;1356847-98-7
|
| PubChem CID |
5484727
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
626.0±55.0 °C at 760 mmHg
|
| 熔点 |
122-123°C
|
| 闪点 |
332.4±31.5 °C
|
| 蒸汽压 |
0.0±1.9 mmHg at 25°C
|
| 折射率 |
1.549
|
| LogP |
3.97
|
| tPSA |
95.94
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
10
|
| 重原子数目 |
31
|
| 分子复杂度/Complexity |
634
|
| 定义原子立体中心数目 |
5
|
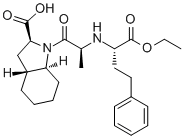
| SMILES |
CCOC(=O)[C@H](CCC1=CC=CC=C1)N[C@@H](C)C(=O)N2[C@H]3CCCC[C@@H]3C[C@H]2C(=O)O
|
| InChi Key |
VXFJYXUZANRPDJ-WTNASJBWSA-N
|
| InChi Code |
InChI=1S/C24H34N2O5/c1-3-31-24(30)19(14-13-17-9-5-4-6-10-17)25-16(2)22(27)26-20-12-8-7-11-18(20)15-21(26)23(28)29/h4-6,9-10,16,18-21,25H,3,7-8,11-15H2,1-2H3,(H,28,29)/t16-,18+,19-,20-,21-/m0/s1
|
| 化学名 |
(2S,3aR,7aS)-1-[(2S)-2-[[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino]propanoyl]-2,3,3a,4,5,6,7,7a-octahydroindole-2-carboxylic
acid
|
| 别名 |
RU 44570 Mavik RU-44570GoptenRU44570 Odrik
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~232.27 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (4.83 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (4.83 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (4.83 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3227 mL | 11.6133 mL | 23.2266 mL | |
| 5 mM | 0.4645 mL | 2.3227 mL | 4.6453 mL | |
| 10 mM | 0.2323 mL | 1.1613 mL | 2.3227 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。