| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
(L)-Tryptophan with plant oils in soft gelatin capsules permitted lower dosage than with usual dosage form. Max of free tryptophan in serum was achieved in 1st hr whereas 4-5 times as much would be required with tablets or hard gelatin capsules. Absorption and Fate. Tryptophan is readily absorbed from the gastro-intestinal tract. Tryptophan is extensively bound to serum albumin. It is metabolized to serotonin and other metabolites, incl kynurenine derivatives, and excreted in the urine. Pyridoxine and ascorbic acid appear to be concerned in its metabolism. Although the free amino acids dissolved in the body fluids are only a very small proportion of the body's total mass of amino acids, they are very important for the nutritional and metabolic control of the body's proteins. ... Although the plasma compartment is most easily sampled, the concentration of most amino acids is higher in tissue intracellular pools. Typically, large neutral amino acids, such as leucine and phenylalanine, are essentially in equilibrium with the plasma. Others, notably glutamine, glutamic acid, and glycine, are 10- to 50-fold more concentrated in the intracellular pool. Dietary variations or pathological conditions can result in substantial changes in the concentrations of the individual free amino acids in both the plasma and tissue pools. /Amino acids/ After ingestion, proteins are denatured by the acid in the stomach, where they are also cleaved into smaller peptides by the enzyme pepsin, which is activated by the increase in stomach acidity that occurs on feeding. The proteins and peptides then pass into the small intestine, where the peptide bonds are hydrolyzed by a variety of enzymes. These bond-specific enzymes originate in the pancreas and include trypsin, chymotrypsins, elastase, and carboxypeptidases. The resultant mixture of free amino acids and small peptides is then transported into the mucosal cells by a number of carrier systems for specific amino acids and for di- and tri-peptides, each specific for a limited range of peptide substrates. After intracellular hydrolysis of the absorbed peptides, the free amino acids are then secreted into the portal blood by other specific carrier systems in the mucosal cell or are further metabolized within the cell itself. Absorbed amino acids pass into the liver, where a portion of the amino acids are taken up and used; the remainder pass through into the systemic circulation and are utilized by the peripheral tissues. /Amino acids/ For more Absorption, Distribution and Excretion (Complete) data for (L)-Tryptophan (9 total), please visit the HSDB record page. Metabolism / Metabolites Hepatic. In Hartnup disease ... tryptophane appear/s/ in urine due to defective renal and intestinal absorption of tryptophane ... It is an intermediary metabolite in the synthesis of serotonin (5-hydroxytryptamine) and 5-hydroxyindole acetic acid (HIAA). Patients with bladder cancer excreted significantly more kynurenic acid, acetylkynurenine, kynurenine, and 3-hydroxykynurenine after ingesting a loading dose of L-tryptophan than did control subjects with no known disease. Tryptophan is metabolized in the liver by tryptophan pyrrolase and tryptophan hydroxylase. Metabolites include hydroxytryptophan, which is then converted to serotonin, and kynurenine derivatives. Some tryptophan is converted to nicotinic acid and nicotinamide. Pyridoxine and ascorbic acid are cofactors in the decarboxylation and hydroxylation, respectively, of tryptophan; pyridoxine apparently prevents the accumulation of the kynurenine metabolites. Yields indole-3-pyruvic acid in man ... and in rats; yields tryptamine in guinea pigs. /From table/ For more Metabolism/Metabolites (Complete) data for (L)-Tryptophan (21 total), please visit the HSDB record page. Hepatic. Biological Half-Life The biological half-life of tryptophan was reported to be 15.8 hr. |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
A number of important side reactions occur during the catabolism of tryptophan on the pathway to acetoacetate. The first enzyme of the catabolic pathway is an iron porphyrin oxygenase that opens the indole ring. The latter enzyme is highly inducible, its concentration rising almost 10-fold on a diet high in tryptophan. Kynurenine is the first key branch point intermediate in the pathway. Kynurenine undergoes deamniation in a standard transamination reaction yielding kynurenic acid. Kynurenic acid and metabolites have been shown to act as antiexcitotoxics and anticonvulsives. A second side branch reaction produces anthranilic acid plus alanine. Another equivalent of alanine is produced further along the main catabolic pathway, and it is the production of these alanine residues that allows tryptophan to be classified among the glucogenic and ketogenic amino acids. The second important branch point converts kynurenine into 2-amino-3-carboxymuconic semialdehyde, which has two fates. The main flow of carbon elements from this intermediate is to glutarate. An important side reaction in liver is a transamination and several rearrangements to produce limited amounts of nicotinic acid, which leads to production of a small amount of NAD+ and NADP+. Interactions Acetylsalicylic acid reduced serum-protein binding of tryptophan in man, causing rise in free serum tryptophan. Changes in metabolic pattern also occurred, with increased urinary excretion of xanthurenic acid and 3-hydroxylkynurenine and decreased excretion of 3-hydroxyanthranilic acid. Although tryptophan has been given to patients receiving MAOIs in the belief that clinical efficacy may be improved, it should be noted that the adverse effects may also be potentiated. Use of tryptophan with drugs that inhibit the reuptake of serotonin may exacerbate the adverse effects of the latter and precipitate the serotonin syndrome. There have been occasional reports of sexual disinhibition in patients taking tryptophan with phenothiazines or benzodiazepines. For more Interactions (Complete) data for (L)-Tryptophan (16 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rat ip 1634 mg/kg LD50 Mouse ip 4800 mg/kg |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Tryptophan is a precursor of serotonin. Because CNS depletion of serotonin is considered to be involved in depression, tryptophan has been used in its treatment. Although it has been given alone, evidence of effectiveness is scant and tryptophan has generally been used as adjunctive therapy in depression. It has sometimes been given with pyridoxine and ascorbic acid, which are involved in its metabolism to serotonin /EXPTL USE/: Inhibition of Walker 256 intramuscular carcinoma in rats by admin of l-tryptophan. (L)-Tryptophan decreases sleep latency and slightly increases sleeping time without altering qualitative characteristics of polygraphic patterns during sleep in normal subjects. In insomniac patients, it increases sleeping time and decreases both sleep latency and number of awakenings. Beneficial effects were observed when L-tryptophan was administered to 2 patients with myoclonus. In each case suspension of methylcellulose and water containing 1 g of (L)-tryptophan/15 mL was prepared and administered orally at a level of 10 g daily in 5 divided doses. For more Therapeutic Uses (Complete) data for (L)-Tryptophan (11 total), please visit the HSDB record page. Drug Warnings Since serotonin plays a role in inducing and maintaining sleep, l-tryptophan has been administered orally to increase brain levels of serotonin. Although a dose of 1 g significantly decreased sleep latency and total time awake without altering sleep patterns, the hypnotic action is observed only during the early part of the sleep cycle, is unpredictable, and is not characterized by a satisfactory dose-response relationship. Because the hypnotic action has not been confirmed in other studies, this use of l-tryptophan must be considered investigational and the drug is not recommended in routine clinical practice. In order to avoid central serotonergic toxicity, tryptophan should not be used in patients also receiving a monoamine oxidase inhibitor or the serotonin uptake inhibitor, fluoxetine (Prozac). Tryptophan-containing products have been associated with the eosinophilia-myalgia syndrome. Other adverse effects that have been reported include nausea, headache, lightheadedness, and drowsiness. An increased incidence of bladder tumours has been reported in mice given l-tryptophan orally as well as in cholesterol pellets embedded in the bladder lumen. However, there was no increase in tumour incidence when only high-dose, oral tryptophan was given. Tryptophan has been associated with eosinophilia-myalgia syndrome; caution is advised in patients receiving the drug who develop some, but not all, of the symptoms of this syndrome. It should not be used in those with a history of eosinophilia-myalgia syndrome associated with tryptophan treatment. For more Drug Warnings (Complete) data for (L)-Tryptophan (7 total), please visit the HSDB record page. Pharmacodynamics Tryptophan is critical for the production of the body's proteins, enzymes and muscle tissue. It is also essential for the production of niacin, the synthesis of the neurotransmitter serotonin and melatonin. Tryptophan supplements can be used as natural relaxants to help relieve insomnia. Tryptophan can also reduce anxiety and depression and has been shown to reduce the intensity of migraine headaches. Other promising indications include the relief of chronic pain, reduction of impulsivity or mania and the treatment of obsessive or compulsive disorders. Tryptophan also appears to help the immune system and can reduce the risk of cardiac spasms. Tryptophan deficiencies may lead to coronary artery spasms. Tryptophan is used as an essential nutrient in infant formulas and intravenous feeding. Tryptophan is marketed as a prescription drug (Tryptan) for those who do not seem to respond well to conventional antidepressants. It may also be used to treat those afflicted with seasonal affective disorder (a winter-onset depression). Tryptopan serves as the precursor for the synthesis of serotonin (5-hydroxytryptamine, 5-HT) and melatonin (N-acetyl-5-methoxytryptamine). |
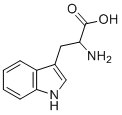
| 分子式 |
C11H12N2O2
|
|---|---|
| 分子量 |
204.22
|
| 精确质量 |
204.089
|
| CAS号 |
73-22-3
|
| 相关CAS号 |
27813-82-7
|
| PubChem CID |
6305
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.4±0.1 g/cm3
|
| 沸点 |
447.9±35.0 °C at 760 mmHg
|
| 熔点 |
289-290 °C (dec.)(lit.)
|
| 闪点 |
224.7±25.9 °C
|
| 蒸汽压 |
0.0±1.1 mmHg at 25°C
|
| 折射率 |
1.698
|
| LogP |
1.04
|
| tPSA |
79.11
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
15
|
| 分子复杂度/Complexity |
245
|
| 定义原子立体中心数目 |
1
|
| SMILES |
C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)O)N
|
| InChi Key |
QIVBCDIJIAJPQS-VIFPVBQESA-N
|
| InChi Code |
InChI=1S/C11H12N2O2/c12-9(11(14)15)5-7-6-13-10-4-2-1-3-8(7)10/h1-4,6,9,13H,5,12H2,(H,14,15)/t9-/m0/s1
|
| 化学名 |
(2S)-2-amino-3-(1H-indol-3-yl)propanoic acid
|
| 别名 |
NSC-13119 NSC 13119 Tryptophan
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: (1). 本产品在运输和储存过程中需避光。 (2). 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~7.69 mg/mL (~37.65 mM)
H2O : ~5 mg/mL (~24.48 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 0.77 mg/mL (3.77 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 7.7 mg/mL澄清的DMSO储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 0.77 mg/mL (3.77 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 7.7 mg/mL 澄清 DMSO 储备液加入 900 μL 20% SBE-β-CD 生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 0.77 mg/mL (3.77 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 6.25 mg/mL (30.60 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶 (<60°C). 配方 5 中的溶解度: 20 mg/mL (97.93 mM) in 50% PEG300 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 需要超声波和加温并加热至 45°C。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.8967 mL | 24.4834 mL | 48.9668 mL | |
| 5 mM | 0.9793 mL | 4.8967 mL | 9.7934 mL | |
| 10 mM | 0.4897 mL | 2.4483 mL | 4.8967 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。