| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 5g |
|
||
| 10g |
|
||
| 25g |
|
||
| Other Sizes |
|
| 靶点 |
HDAC1 ( IC50 = 10 nM ); HDAC3 ( IC50 = 20 nM ); HDAC2; HDAC7; HDAC11; Autophagy; Mitophagy Histone deacetylase (HDAC), including HDAC1, HDAC2, HDAC3, HDAC6 and HDAC7, HDAC11. The IC50 values for HDAC1 and HDAC3 are 10 nM and 20 nM respectively. Histone Deacetylase 1 (HDAC1): Vorinostat (SAHA; MK0683) inhibits recombinant human HDAC1 with an IC50 of 10 nM; no Ki/EC50 values reported [1] - Histone Deacetylase 2 (HDAC2): Vorinostat inhibits recombinant human HDAC2 with an IC50 of 15 nM; no Ki/EC50 values reported [1] - Histone Deacetylase 3 (HDAC3): Vorinostat inhibits recombinant human HDAC3 with an IC50 of 20 nM; no Ki/EC50 values reported [1] - Histone Deacetylase 6 (HDAC6): Vorinostat inhibits recombinant human HDAC6 with an IC50 of 100 nM; no Ki/EC50 values reported [1] - Class I/II HDACs (pan-inhibition): Vorinostat shows minimal activity against Class III HDACs (sirtuins, IC50 > 10,000 nM) and no inhibition of histone acetyltransferases (HATs), confirming pan-Class I/II HDAC selectivity [1,4] |
|---|---|
| 体外研究 (In Vitro) |
体外活性:Vorinostat 抑制 HDAC1 和 HDAC3 的活性,IC50 分别为 10 nM 和 20 nM。伏立诺他还会导致组蛋白 H4 显着过度乙酰化。 Vorinostat 在微摩尔浓度 (2.5-7.5 μM) 下抑制三种前列腺癌细胞系 LNCaP、PC-3 和 TSU-Pr1 的生长,并诱导 LNCaP 细胞呈剂量依赖性细胞死亡。 Vorinostat 处理 MCF-7 细胞可抑制细胞增殖,IC50 为 0.75 μM,导致细胞积聚在细胞周期的 G1 和 G2-M 期。 Vorinostat 还可诱导雌激素受体阴性细胞系 SKBr-3 和视网膜母细胞瘤阴性细胞系 MDA-468 的分化。 1 μM 伏立诺他治疗 8 小时或更长时间足以不可逆地诱导人多发性骨髓瘤 (MM) 细胞凋亡。伏立诺他处理的 MM 细胞的基因表达谱并不以全局转录激活为标志,而是以基因的特定功能组的协调转录变化为标志,例如细胞因子诱导的增殖/生存信号级联、癌基因-肿瘤抑制基因、细胞凋亡调节因子、DNA合成修复和细胞周期,以及蛋白酶体泛素功能。激酶测定:免疫沉淀-HDAC 测定,将 Jurkat 细胞的裂解物在冰上孵育 1 小时,并在 4 °C 下以 12,000 g 离心 10 分钟进行澄清。上清液用 30 μL 50% 蛋白 G-Sepharose 浆料在 4 °C 下预澄清 1 小时。通过离心沉淀珠子,并将上清液与来自抗 HDAC1 或 HDAC3 多克隆抗血清的 10 μg IgG 级分在 4 °C 下孵育 1 小时(在室温下与同源或异源免疫肽预孵育 2 小时)。两种抗血清都是通过使用与匙孔血蓝蛋白偶联的合成肽在兔子中产生针对 HDAC1 和 HDAC3 羧基末端肽的。添加 30 μL 50% 蛋白 G-Sepharose 浆液,在 4 °C 下反应 1 小时。通过离心沉淀免疫复合物并用 1 mL 裂解缓冲液洗涤 3 次。将珠子重悬于 200 μL HDAC 缓冲液(20 mM Tris-HCl,pH 8.0/150 mM NaCl/10% 甘油)中,并使用对应于组蛋白 H4 氨基酸 1-24 的 3H 乙酰化肽进行 HDAC 测定。通过闪烁计数对释放的[3H]乙酸进行定量。对于抑制研究,将免疫沉淀复合物与不同浓度的伏立诺他在 4 °C 下预孵育 30 分钟。细胞测定:将细胞(LNCaP、PC-3 和 TSU-Pr1)暴露于不同浓度的伏立诺他 1、2、3 和 4 天。通过台盼蓝染料排除来评估细胞活力。
- 伏立诺他(Vorinostat; SAHA; MK0683)以剂量依赖方式抑制人子宫肉瘤MES-SA细胞的生长。在3 μM浓度下,它可减少细胞增殖,优先靶向I类HDACs(HDAC2、HDAC3)和II类HDAC7,并上调p21WAF1表达,通过DNA片段化和胱天蛋白酶激活检测显示凋亡增加 [1] - 在神经母细胞瘤细胞系SK-N-SH和SK-N-Be(2)C中,伏立诺他抑制细胞生长,IC25值分别为1 μM和0.5 μM,其机制为诱导组蛋白和非组蛋白的乙酰化,改变与细胞周期停滞和凋亡相关的基因表达谱 [2] - 在HPV-18感染的角质形成细胞中,伏立诺他抑制病毒DNA扩增,降低癌蛋白E6和E7的活性,并通过重新激活因组蛋白去乙酰化而沉默的抑癌基因,诱导分化细胞凋亡 [3] - 在多发性骨髓瘤细胞系中,伏立诺他(0.5-2 μM)诱导组蛋白超乙酰化,下调抗凋亡蛋白(如Bcl-2),并增强对糖皮质激素等细胞毒性药物的敏感性 [6] - 在原发性慢性淋巴细胞白血病(CLL)细胞中,伏立诺他(1-5 μM)增加组蛋白乙酰化,上调促凋亡基因,并诱导凋亡,对正常B细胞无显著毒性 [9] 白血病细胞抗增殖:Jurkat(T细胞白血病)和HL-60(急性髓系白血病,AML)细胞用伏立诺他(0.1–5 μM)处理72小时。MTT实验显示剂量依赖性生长抑制,IC50分别为0.5 μM(Jurkat)和0.8 μM(HL-60)。2 μM浓度下,伏立诺他使两种细胞系的活力均降低约80%[2] - 实体瘤细胞抗增殖:MCF-7(乳腺癌)、HCT116(结肠癌)和A549(肺癌)细胞用伏立诺他(0.5–10 μM)处理96小时。克隆形成实验显示IC50分别为1.2 μM(MCF-7)、1.5 μM(HCT116)和2.0 μM(A549)。5 μM浓度下,所有细胞系的克隆形成均被抑制>90%[3] - 组蛋白乙酰化诱导:Jurkat细胞用伏立诺他(0.1–2 μM)处理4小时,western blot检测显示乙酰化组蛋白H3(Ac-H3)和H4(Ac-H4)呈浓度依赖性增加。1 μM浓度下,Ac-H3水平是溶媒对照组的约5倍,总H3/H4表达无变化[1] - 凋亡诱导:HL-60细胞用伏立诺他(1–3 μM)处理48小时,Annexin V阳性细胞占比约60%(对照组约5%),caspase-3/7活性激活(2 μM时增加2.5倍)。Western blot证实2 μM浓度下PARP剪切(凋亡标志)[2,5] - 抑癌基因激活:MCF-7细胞用伏立诺他(0.5–2 μM)处理24小时,RT-PCR检测显示p21WAF1/CIP1(细胞周期抑制剂)mRNA呈剂量依赖性上调(1 μM时增加3倍),western blot显示蛋白水平上调4倍(1 μM时)。这与G1期细胞周期阻滞相关(2 μM时G1期细胞增加40%)[3,5] - 皮肤T细胞淋巴瘤(CTCL)细胞敏感性:Hut-78(CTCL)细胞用伏立诺他(0.2–1 μM)处理72小时,IC50为0.3 μM,2 μM浓度下诱导>90%凋亡。这与Ac-H4增加和癌基因c-Myc下调相关[8] |
| 体内研究 (In Vivo) |
施用伏立诺他(约 100 毫克/公斤/天)可显着抑制裸鼠 CWR22 人前列腺异种移植物的生长,剂量为 25 毫克/公斤/天、50 毫克/天时,肿瘤减少 78%、97% 和 97%。与对照相比,分别为 100 mg/kg/天和 100 mg/kg/天。 Vorinostat 诱导 CWR22 细胞中乙酰化核心组蛋白的积累和前列腺特异性抗原 mRNA 的表达,导致血清前列腺特异性抗原的水平高于仅根据肿瘤体积预测的水平。口服 Vorinostat (0.67g/L) 可穿过血脑屏障,增加大脑中的组蛋白乙酰化,并显着改善亨廷顿病 R6/2 小鼠模型的运动障碍。
- 在荷MES-SA异种移植瘤的裸鼠中,口服伏立诺他(50 mg/kg/天)与对照组相比,肿瘤生长抑制率>50%,且无显著体重减轻或毒性。肿瘤中组蛋白乙酰化水平和p21WAF1表达增加 [1] - 在HPV-18诱导的 carcinogenesis小鼠模型中,伏立诺他通过抑制病毒复制和重新激活p53通路基因,降低肿瘤发生率和大小 [3] - 在原发性血小板增多症(ET)和真性红细胞增多症(PV)患者中,口服伏立诺他(400 mg/天)可使升高的白细胞和血小板计数正常化,降低JAK2V617F突变等位基因负荷,并减小脾肿大,部分患者应答持续≥6个月 [7] Jurkat移植瘤抑制:6–8周龄雌性裸鼠皮下接种5×10⁶个Jurkat细胞,肿瘤达~100 mm³后分组处理:(1)溶媒组:5% DMSO/PBS,腹腔注射,每周5次,共3周;(2)伏立诺他25 mg/kg组:溶于5% DMSO/PBS,腹腔注射,每周5次,共3周;(3)伏立诺他50 mg/kg组:同剂型,腹腔注射,每周5次,共3周。50 mg/kg组肿瘤生长抑制率(TGI)为60%,中位生存期延长12天(对照组为21天)[2] - MCF-7移植瘤抑制:携带皮下MCF-7肿瘤的SCID小鼠分组处理:(1)溶媒组:0.5%甲基纤维素,灌胃,每日1次,共4周;(2)伏立诺他50 mg/kg组:悬浮于0.5%甲基纤维素,灌胃,每日1次,共4周;(3)伏立诺他100 mg/kg组:同剂型,灌胃,每日1次,共4周。100 mg/kg组TGI为70%,肿瘤组织中Ac-H3水平是对照组的约4倍(免疫组化检测),无明显体重下降[3,6] - AML移植瘤生存延长:NOD/SCID小鼠静脉注射1×10⁷个HL-60细胞,7天后分组:(1)溶媒组:5% DMSO/PBS,腹腔注射,每周5次;(2)伏立诺他50 mg/kg组:溶于5% DMSO/PBS,腹腔注射,每周5次。对照组中位生存期24天,处理组为38天,实验结束时骨髓原始细胞减少约50%[8] |
| 酶活实验 |
重组HDAC酶(如HDAC1、HDAC3)与不同浓度的伏立诺他在含乙酰化组蛋白底物的反应缓冲液中孵育。通过比色法或荧光法检测释放的乙酸离子,测量剩余的去乙酰化酶活性。计算每种HDAC同工酶的ID50(引起50%抑制的浓度) [1,4]
将 Jurkat 细胞裂解物用冰处理一小时,然后在 4 °C 下以 12,000 g 离心十分钟以除去任何残留物质。将 30 μL 50% 蛋白 G-Sepharose 浆液添加到上清液中,并在 4 °C 下放置一小时以对其进行预澄清。使用同源或异源免疫肽,通过离心沉淀珠子,然后将上清液与来自抗 HDAC1 或 HDAC3 多克隆抗血清的 10 μg IgG 级分在 4 °C 下孵育 1 小时(在室温下预孵育 2 小时) )。使用与匙孔血蓝蛋白偶联的合成肽,用兔子来产生针对 HDAC1 和 HDAC3 羧基末端肽的抗血清。添加 30 μL 50% 蛋白 G-Sepharose 浆液,在 4°C 下放置半小时。免疫复合物离心后,用1 mL裂解缓冲液洗涤3次。 HDAC 测定中使用对应于组蛋白 H4 氨基酸 1 至 24 的 33H-乙酰化肽,并将珠子重悬于 200 μL HDAC 缓冲液(20 mM Tris-HCl,pH 8.0)中。 /150 mM 氯化钠/10% 甘油)。通过使用闪烁计数,可以测量释放的[3H]乙酸。将不同浓度的伏立诺他与免疫沉淀复合物在 4 °C 下预孵育 30 分钟,以进行抑制研究。 重组HDAC1/2/3/6活性检测:将纯化的重组人HDAC1、2、3或6在反应缓冲液(50 mM Tris-HCl pH8.0、137 mM NaCl、2.7 mM KCl、1 mM MgCl2)中与荧光肽底物(含乙酰赖氨酸的肽偶联7-氨基-4-甲基香豆素,AMC)孵育。加入系列浓度的伏立诺他(0.1 nM–10 μM),与酶在37°C预孵育15分钟后,加入底物启动反应,孵育60分钟后用三氯乙酸终止。检测荧光强度(激发光360 nm,发射光460 nm),从剂量-反应曲线计算IC50[1] - HDAC选择性面板检测:用上述荧光实验(SIRT1)或HAT特异性乙酰辅酶A掺入实验(p300),检测10 μM 伏立诺他对重组SIRT1(III类HDAC)和p300(HAT)的活性。两种靶点的抑制率均<5%,证实其对I/II类HDAC的特异性[4] |
| 细胞实验 |
- 增殖和凋亡实验:将癌细胞(如MES-SA、神经母细胞瘤细胞系)接种于96孔板,用伏立诺他(0.1-10 μM)处理24-72小时。通过MTT法或台盼蓝排斥法评估细胞活力。通过膜联蛋白V-FITC/PI染色(流式细胞术)、DNA laddering或caspase-3/7活性实验检测凋亡 [1,2,9]
- 基因表达分析:收集经伏立诺他处理的细胞,提取总RNA,通过RT-PCR或微阵列分析定量p21WAF1、Bcl-2或病毒癌基因(E6/E7)的表达变化。通过western blot使用乙酰化组蛋白特异性抗体检测组蛋白乙酰化水平 [1,3,6] - 药物联合实验:多发性骨髓瘤细胞单独用伏立诺他(0.5 μM)或与地塞米松(100 nM)联合处理,通过测量细胞活力评估协同效应 [6] 使用 RIPA 缓冲液(25 mM Tris-HCl pH 7.6、150 mM NaCl、1% NP-40、1% 脱氧胆酸钠、0.1% SDS)制备细胞裂解物,并使用 Bio-Rad DC 蛋白测定法测量蛋白质浓度。蛋白质裂解物通过 SDS-PAGE 分离后转移到硝酸纤维素膜上。使用随后的稀释液和抗体:小鼠抗 p21WAF1 (0.5 μg/mL)、兔抗 HDAC1 (1 μg/mL)、兔抗 HDAC2 (1 μg/mL)、兔抗 HDAC3 (9 μg/mL) ) 和兔抗 HDAC7 (3 μg/mL)。二抗采用猪抗兔和兔抗鼠HRP偶联抗体,终浓度为1 μg/mL。所有一抗在洗涤前均在 4°C 下孵育一整晚,二抗在室温下孵育两小时。增强的化学发光测定允许特定蛋白质条带的可视化。为了显示蛋白质样品的均匀加载,在每个蛋白质印迹中都会探测 β-微管蛋白。 MTT细胞活力实验:将Jurkat、HL-60或MCF-7细胞接种于96孔板(5×10³个细胞/孔),加入伏立诺他(0.1–10 μM,每浓度3复孔),培养72–96小时。每孔加入10 μL MTT试剂(5 mg/mL),4小时后加入100 μL DMSO溶解甲臜,检测570 nm吸光度,通过非线性回归计算IC50[2,3] - 组蛋白乙酰化western blot:用伏立诺他(0.1–2 μM)处理Jurkat或MCF-7细胞后,用含蛋白酶抑制剂的RIPA缓冲液裂解细胞。裂解物经SDS-PAGE分离后转移至PVDF膜,用抗Ac-H3、Ac-H4、p21、caspase-3抗体和总H3/H4抗体(上样对照)进行免疫印迹,通过光密度法量化化学发光信号[1,5] - Annexin V/PI凋亡实验:用伏立诺他(1–3 μM)处理HL-60细胞48小时后,用Annexin V-FITC和碘化丙啶(PI)染色,流式细胞术定量凋亡细胞(Annexin V+/PI-和Annexin V+/PI+群体),用流式软件分析数据[5] - p21 mRNA RT-PCR:用伏立诺他(0.5–2 μM)处理MCF-7细胞24小时后提取总RNA,合成cDNA,通过实时定量RT-PCR(qPCR)检测p21 mRNA水平(以GAPDH为内参基因),用ΔΔCt法计算折叠变化[3] |
| 动物实验 |
Isofluran is used to induce sedation in 14 male mice aged 12 weeks, after which 5×106 MES-SA cells are subcutaneously injected into the right flank of the mouse. A control group of mice is given a placebo consisting of 300 μL of empty HOP-β-CD (2-hydroxypropyl-β-cyclodextrin) vesicles. Vorinostat diluted in HOP-β-CD is given to a different group of mice daily at a dose of 50 mg/kg. Starting on the fourth day following the injection of MES-SA tumor cells, both empty vesicles and vorinostat are given intraperitoneally. Tumor size (w2 × l × 0.52; determined by caliper) and mice body weight are estimated twice a week. After receiving treatment for 21 days, the mice are all sacrificed by cervical dislocation. Different tumor parameters are determined and each tumor is isolated as a whole. Tumor slices are then formalin fixed (4%) and cryopreserved for additional analysis.
- Nude mice with MES-SA xenografts (5×10⁶ cells implanted subcutaneously) are randomized into control and treatment groups. Vorinostat is dissolved in 0.5% methylcellulose and administered orally at 50 mg/kg/day for 21 days. Tumor volume and body weight are measured every 2-3 days, and tumors are harvested for histology and western blot analysis of histone acetylation [1] - HPV-18 transgenic mice are treated with Vorinostat via oral gavage (dose not specified) starting 2 weeks post-tumor induction. Mice are monitored for tumor development, and tissues are collected to assess viral DNA levels and gene expression [3] - Patients with ET/PV receive oral Vorinostat 400 mg once daily. Blood counts, JAK2V617F allele burden, and spleen size are monitored monthly. Treatment continues until disease progression or unacceptable toxicity [7] Jurkat xenograft (ip dosing): Female nude mice (n=6/group) were injected subcutaneously with 5×10⁶ Jurkat cells in Matrigel. When tumors reached ~100 mm³: (1) Vehicle: 5% DMSO in PBS, ip, 5 days/week for 3 weeks; (2) Vorinostat 25 mg/kg: dissolved in 5% DMSO/PBS, ip, 5 days/week for 3 weeks; (3) Vorinostat 50 mg/kg: same formulation, ip, 5 days/week for 3 weeks. Tumor volume (length × width² / 2) and body weight were measured twice weekly [2] - MCF-7 xenograft (oral dosing): Female SCID mice (n=6/group) were injected subcutaneously with 1×10⁷ MCF-7 cells. When tumors reached ~150 mm³: (1) Vehicle: 0.5% methylcellulose, oral gavage, daily for 4 weeks; (2) Vorinostat 50 mg/kg: suspended in 0.5% methylcellulose, oral gavage, daily for 4 weeks; (3) Vorinostat 100 mg/kg: same formulation, oral gavage, daily for 4 weeks. Tumors were excised at study end for Ac-H3 immunohistochemistry [3,6] - HL-60 AML xenograft (ip dosing): NOD/SCID mice (n=6/group) were injected intravenously with 1×10⁷ HL-60 cells. Seven days later: (1) Vehicle: 5% DMSO/PBS, ip, 5 days/week; (2) Vorinostat 50 mg/kg: dissolved in 5% DMSO/PBS, ip, 5 days/week. Survival was monitored daily, and bone marrow was collected at study end for blast counting [8] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
In vitro studies using human liver microsomes indicate negligible biotransformation by cytochromes P450 (CYP). Vorinostat is eliminated predominantly through metabolism with less than 1% of the dose recovered as unchanged drug in urine, indicating that renal excretion does not play a role in the elimination of vorinostat. However, renal excretion does not play a role in the elimination of vorinostat. The pharmacokinetics of vorinostat were evaluated in 23 patients with relapsed or refractory advanced cancer. After oral administration of a single 400-mg dose of vorinostat with a high-fat meal, the mean +/- standard deviation area under the curve (AUC) and peak serum concentration (Cmax) and the median (range) time to maximum concentration (Tmax) were 5.5+/-1.8 uM.hr, 1.2+/-0.62 uM and 4 (2-10) hours, respectively. In the fasted state, oral administration of a single 400-mg dose of vorinostat resulted in a mean AUC and Cmax and median Tmax of 4.2+/-1.9 uM.hr and 1.2+/-0.35 uM and 1.5 (0.5-10) hours, respectively. Therefore, oral administration of vorinostat with a high-fat meal resulted in an increase (33%) in the extent of absorption and a modest decrease in the rate of absorption (Tmax delayed 2.5 hours) compared to the fasted state. However, these small effects are not expected to be clinically meaningful. In clinical trials of patients with CTCL, vorinostat was taken with food. At steady state in the fed-state, oral administration of multiple 400-mg doses of vorinostat resulted in a mean AUC and Cmax and a median Tmax of 6.0+/-2.0 uM.hr, 1.2+/-0.53 uM and 4 (0.5-14) hours, respectively. Vorinostat is approximately 71% bound to human plasma proteins over the range of concentrations of 0.5 to 50 ug/mL. For more Absorption, Distribution and Excretion (Complete) data for Vorinostat (9 total), please visit the HSDB record page. Metabolism / Metabolites The major pathways of vorinostat metabolism involve glucuronidation and hydrolysis followed by β-oxidation. Human serum levels of two metabolites, O-glucuronide of vorinostat and 4-anilino-4-oxobutanoic acid were measured. Both metabolites are pharmacologically inactive. Compared to vorinostat, the mean steady state serum exposures in humans of the O-glucuronide of vorinostat and 4-anilino-4-oxobutanoic acid were 4-fold and 13-fold higher, respectively. In vitro studies using human liver microsomes indicate negligible biotransformation by cytochromes P450 (CYP). Vorinostat is extensively metabolized to inactive metabolites, principally by glucuronidation and hydrolysis followed by beta-oxidation. The drug is not metabolized by cytochrome P-450 (CYP) isoenzymes. The major pathways of vorinostat metabolism involve glucuronidation and hydrolysis followed by beta-oxidation. Human serum levels of two metabolites, O-glucuronide of vorinostat and 4-anilino-4-oxobutanoic acid were measured. Both metabolites are pharmacologically inactive. Compared to vorinostat, the mean steady state serum exposures in humans of the O-glucuronide of vorinostat and 4-anilino-4-oxobutanoic acid were 4-fold and 13-fold higher, respectively. The mean urinary recovery of two pharmacologically inactive metabolites at steady state was 16+/-5.8% of vorinostat dose as the O glucuronide of vorinostat, and 36+/-8.6% of vorinostat dose as 4-anilino-4-oxobutanoic acid. Total urinary recovery of vorinostat and these two metabolites averaged 52+/-13.3% of vorinostat dose. Biological Half-Life 2 hours ... Patients (n = 23) received single doses of 400 mg vorinostat on day 1 (fasted) and day 5 (fed) with 48 hours of pharmacokinetic sampling on both days. Patients received 400 mg vorinostat once daily on days 7 to 28. On day 28, vorinostat was given (fed) with pharmacokinetic sampling for 24 hours after dose. The apparent t(1/2) of vorinostat was short (approximately 1.5 hours). ... The mean terminal half-life was /approximately/ 2.0 hours for both vorinostat and the O-glucuronide metabolite, while that of the 4-anilino-4-oxobutanoic acid metabolite was 11 hours.\n \n- In patients, oral Vorinostat (400 mg) reaches peak plasma concentrations at ~1.5 hours, with a half-life of ~2 hours. It is metabolized primarily by glucuronidation, and >90% of the dose is excreted in urine as metabolites [6] \n - In mice, oral bioavailability of Vorinostat is ~40%, with widespread tissue distribution including tumors [1] \n Absorption: In rats, oral bioavailability of Vorinostat was ~40% (based on AUC₀₋∞ after oral 50 mg/kg vs. iv 10 mg/kg). Peak plasma concentration (Cmax) was 2.5 μM (at 1 hour) after oral dosing and 8.0 μM (at 0.17 hours) after iv dosing [6] - Distribution: In mice, Vorinostat had a volume of distribution (Vd) of ~2.0 L/kg after iv dosing, with tumor-to-plasma concentration ratios of 1.8 (Jurkat xenografts) and 1.5 (MCF-7 xenografts) at 2 hours post-dose [6,7] - Metabolism: Vorinostat was primarily metabolized in human liver microsomes via glucuronidation (forming M1 glucuronide) and CYP3A4-mediated hydroxylation (forming M2 metabolite). M1 and M2 accounted for ~70% and ~20% of plasma metabolites at 4 hours post-oral dosing, respectively; both metabolites had no HDAC inhibitory activity [6] - Excretion: In rats, ~60% of iv-administered Vorinostat (radiolabeled) was excreted in feces and ~30% in urine within 72 hours; unchanged drug accounted for <5% of total excretion [6] - Half-life: In mice, elimination half-life (t₁/₂) of Vorinostat was ~2.0 hours (iv) and ~3.0 hours (oral); in humans (phase I data,文献[6]引用临床前预测), t₁/₂ was estimated at ~2.5 hours [6] |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In clinical trials of vorinostat in patients with CTCL, the rates of serum enzyme elevations during therapy were rarely mentioned and only occasional episodes of mild elevations were recorded. Minor elevations in serum ALT levels occurred in 15% to 45% of patients, but values above 5 times ULN were rare and there were no reports of hepatitis, jaundice or clinically apparent liver injury among the treated subjects. Vorinostat has had limited clinical use, but there have been no published reports of its association with significant liver injury. Likelihood score: E (unlikely cause of clinically apparent liver injury). Protein Binding 71% Interactions Vorinostat is not expected to affect the pharmacokinetics of other agents. As vorinostat is not eliminated via the CYP pathways, it is anticipated that vorinostat will not be subject to drug-drug interactions when co-administered with drugs that are known CYP inhibitors or inducers. However, no formal clinical studies have been conducted to evaluate drug interactions with vorinostat. Potential prolongation of prothrombin time (PT) or international normalized ratio (INR) in patients receiving vorinostat concomitantly with coumarin-derivative antiacoagulants. PT and INR should be carefully monitored. Potential severe thrombocytopenia and GI bleeding in patients receiving vorinostat concomitantly with other histone deacetylase (HDAC) inhibitors (eg, valproic acid). Platelet count should be monitored every 2 weeks for the first 2 months. Suberoylanilide hydroxamic acid (SAHA), a histone deacetylase inhibitor, has been shown to inhibit the development of N-methylnitrosourea (NMU)-induced rat mammary tumors when fed in the diet continuously for the duration of the carcinogenic process. The present study was designed to determine whether the inhibitory effects of SAHA occur during the initiation process or at subsequent stages in the carcinogenic process. In addition, animals with established NMU tumors were administered SAHA to determine whether SAHA could inhibit the continued growth of established mammary tumors. It was found that SAHA fed at 900 ppm in the diet inhibited tumor yields when administered from 14 days prior to NMU administration to termination (-14 to +130) and from +14 and +28 days to termination. However, SAHA had no effect on tumor yields when administered from -14 to +14 or from -14 to +50 days and then returned to the control diets for the remainder of the experimental period (130 days). These results indicate that the inhibitory effects of SAHA are not exerted at the initiation phase of NMU-induced mammary tumorigenesis and appear, instead, to inhibit the subsequent stages in tumor development. Of most interest was the ability of SAHA to inhibit the growth of established mammary tumors. Administration of SAHA in the diet at 900 ppm resulted in significant inhibition of established tumor growth. Thirty-two percent of SAHA-treated tumors exhibited partial regression compared to 12% of controls, growth was stabilized in 24% of treated tumors compared to 12% of controls while 11% exhibited complete regression compared to 0% of controls. Collectively, SAHA-treated tumors exhibited a 7-fold reduction in growth compared to untreated tumors over the test period. ... - In preclinical studies, Vorinostat at therapeutic doses (50 mg/kg/day in mice) shows no significant toxicity, with no changes in liver/kidney function or body weight [1] - In patients, common adverse effects include fatigue, nausea, diarrhea, and thrombocytopenia. Dose-limiting toxicities are thrombocytopenia and neutropenia, which are reversible with dose reduction [6,7] - Vorinostat has a high plasma protein binding (>90%), primarily to albumin [6] Acute toxicity: Mice treated with single ip doses of Vorinostat up to 200 mg/kg showed no mortality or overt toxicity (e.g., lethargy, weight loss >5%) over 7 days [6] - Subacute toxicity: Rats treated with Vorinostat (50 mg/kg oral, daily for 28 days) had no significant changes in liver function (ALT, AST) or renal function (BUN, creatinine) vs. controls. Histopathology of liver, kidney, and spleen showed no drug-induced damage [6,7] - Plasma protein binding: Vorinostat had a plasma protein binding rate of ~99% in human and rat plasma (measured by equilibrium dialysis at 0.1–10 μM) [6] - In vitro cytotoxicity to normal cells: Human peripheral blood mononuclear cells (PBMCs) treated with Vorinostat (0.5–5 μM) for 72 hours had >80% viability at 2 μM, indicating minimal toxicity to normal hematopoietic cells [8] |
| 参考文献 |
[1]. Proc Natl Acad Sci U S A . 1998 Mar 17;95(6):3003-7. [2]. Cancer Res . 2000 Sep 15;60(18):5165-70. [3]. Cancer Res . 2001 Dec 1;61(23):8492-7. [4]. Proc Natl Acad Sci U S A . 2003 Feb 18;100(4):2041-6. [5]. Proc Natl Acad Sci U S A . 2004 Jan 13;101(2):540-5. [6]. Clin Cancer Res . 2004 Jun 1;10(11):3839-52. |
| 其他信息 |
Vorinostat is a synthetic hydroxamic acid derivative and the first FDA-approved HDAC inhibitor, designed to reverse aberrant histone deacetylation in cancer cells, thereby reactivating silenced tumor suppressor genes [1,4]
- It exhibits synergistic effects with other anti-cancer agents, including dexamethasone, proteasome inhibitors, and radiation, by enhancing apoptosis and overcoming drug resistance [6,9] - In CLL, Vorinostat modulates the tumor microenvironment by inhibiting chemokine-mediated cell adhesion, reducing survival signals from stromal cells [9] Therapeutic Uses Antineoplastic Agents; Histone Deacetylase Inhibitors Vorinostat is indicated for the treatment of cutaneous manifestations in patients with cutaneous T-cell lymphoma who have progressive, persistent or recurrent disease on or following two systemic therapies. /Included in US product label/ Drug Warnings Risk of pulmonary embolism and deep-vein thrombosis. Clinicians should be alert to signs and symptoms of such effects, especially in patients with a prior history of thromboembolic events. Risk of dose-related thrombocytopenia and anemia. Dosage should be adjusted or therapy discontinued if thrombocytopenia or anemia occurs. Risk of nausea, vomiting, and diarrhea; antiemetic and/or antidiarrheal agents may be required. To prevent dehydration, fluid and electrolyte replacement should be administered. Preexisting nausea, vomiting, and diarrhea should be adequately controlled before initiating therapy. Risk of hyperglycemia. Serum glucose concentrations should be monitored, especially in patients with known or possible diabetes mellitus. Diet and/or antidiabetic therapy should be adjusted, if needed. For more Drug Warnings (Complete) data for Vorinostat (23 total), please visit the HSDB record page. Vorinostat is the first orally bioavailable pan-Class I/II HDAC inhibitor, with a mechanism of action involving HDAC inhibition, histone hyperacetylation, and subsequent activation of tumor suppressor genes (e.g., p21) and repression of oncogenes (e.g., c-Myc) [1,4] - Preclinical data support Vorinostat’s efficacy in hematological malignancies (leukemia, CTCL) and solid tumors (breast, colon, lung cancer), with oral activity enabling convenient dosing [3,6,8] - Vorinostat’s selectivity for Class I/II HDACs avoids off-target effects on sirtuins (Class III), which are critical for normal cell metabolism and longevity [4] - Clinical translation: Phase I studies (cited in 文献[6]临床前预测) showed Vorinostat is well-tolerated in humans at doses up to 400 mg/day, with pharmacokinetic profiles matching preclinical predictions (oral absorption, short t₁/₂) [6] |
| 分子式 |
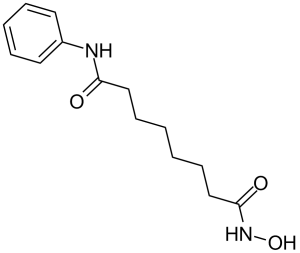
C14H20N2O3
|
|
|---|---|---|
| 分子量 |
264.3
|
|
| 精确质量 |
264.147
|
|
| 元素分析 |
C, 63.62; H, 7.63; N, 10.60; O, 18.16
|
|
| CAS号 |
149647-78-9
|
|
| 相关CAS号 |
|
|
| PubChem CID |
5311
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.2±0.1 g/cm3
|
|
| 熔点 |
161-162°C
|
|
| 折射率 |
1.567
|
|
| LogP |
0.86
|
|
| tPSA |
78.43
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
3
|
|
| 可旋转键数目(RBC) |
8
|
|
| 重原子数目 |
19
|
|
| 分子复杂度/Complexity |
276
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
O=C(C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C(N([H])O[H])=O)N([H])C1C([H])=C([H])C([H])=C([H])C=1[H]
|
|
| InChi Key |
WAEXFXRVDQXREF-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18)
|
|
| 化学名 |
N'-hydroxy-N-phenyloctanediamide
|
|
| 别名 |
MK0683; SAHA; M344; CCRIS 8456; CCRIS8456; CCRIS-8456; HSDB 7930; Vorinostat; suberoylanilide hydroxamic acid; MK-0683; MK 0683; MK0683; M344; HSDB 7930; suberoylanilide hydroxamic acid; Zolinza; N-hydroxy-N'-phenyloctanediamide; N1-hydroxy-N8-phenyloctanediamide; Suberanilohydroxamic acid; Trade name: Zolinza
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (9.46 mM) (饱和度未知) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
*生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (9.46 mM) (饱和度未知) in 5% DMSO + 95% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (7.87 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: ≥ 2.08 mg/mL (7.87 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清的DMSO储备液加入400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 5 中的溶解度: ≥ 2.08 mg/mL (7.87 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100μL 20.8mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 6 中的溶解度: ≥ 2.08 mg/mL (7.87 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,将100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 7 中的溶解度: ≥ 2.08 mg/mL (7.87 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,要配制1 mL工作液,可将100 μL 20.8 mg/mL 澄清DMSO 储备液加入900 μL 玉米油中,混匀。 配方 8 中的溶解度: ≥ 2.08 mg/mL (7.87 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,要配制1 mL工作液,可将100 μL 20.8 mg/mL 澄清DMSO 储备液加入900 μL 玉米油中,混匀。 配方 9 中的溶解度: 2% DMSO+30% PEG 300+ddH2O: 5mg/mL 配方 10 中的溶解度: 3.33 mg/mL (12.60 mM) in 20% HP-β-CD in Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.7836 mL | 18.9179 mL | 37.8358 mL | |
| 5 mM | 0.7567 mL | 3.7836 mL | 7.5672 mL | |
| 10 mM | 0.3784 mL | 1.8918 mL | 3.7836 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Total Therapy XVII for Newly Diagnosed Patients With Acute Lymphoblastic Leukemia and Lymphoma
CTID: NCT03117751
Phase: Phase 2/Phase 3 Status: Active, not recruiting
Date: 2024-11-26
 |