| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
Influenza A(IC50= 0.95 nM);Influenza B(IC50= 2.7 nM)
|
|---|---|
| 体外研究 (In Vitro) |
Zanamivir(GR-121167X;GG-167;Relenza)与神经氨酸酶活性位点的一组氨基酸相互作用,这些氨基酸在所有甲型和乙型流感病毒株中都是保守的。扎那米韦阻断神经氨酸酶的作用,从而防止细胞受体上唾液酸的裂解,从而防止新形成的病毒颗粒的释放和扩散[2]。
|
| 体内研究 (In Vivo) |
扎那米韦 (GR-121167X; GG-167; Relenza) 口服生物利用度较差,仅为 4-17%。扎那米韦的口服给药一直是一个问题,因为其强亲水性限制了其穿过肠上皮的转运。胆酸钠、羟丙基β-环糊精等渗透促进剂可与扎那米韦合用以增强肠道通透性[3]。
|
| 动物实验 |
Rats
Combinations Rats kept conscious are given IV-R (reference Zanamivir saline solution for intravenous injection) at a dose of 1 mg/kg.PO-SC (Zanamivir with SC for p.o.) and PO-C (Zanamivir control solution for p.o.) are given oral Zanamivir doses of 10 mg/kg. Before, at 0.5, 1, 2, 3, 4, 6, 8, and 24 hours after administration, blood samples are taken. Three rats from each group are sacrificed at each sampling point to remove the lungs following blood collection.After the rats' lungs are removed through a chest incision, the lungs are cleaned with saline. After that, the lungs are placed in an E-tube and kept in a freezer at -80°C until they are examined. After being centrifuged at 1,500 × g for 10 minutes, plasma samples are collected and kept at -20°C until analysis. Zanamivir is analyzed using the previously stated LC-MS/MS method in both plasma and lungs[3]. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Absolute bioavailability is very low following oral administration (2%). Following oral inhalation, bioavailability is 4% to 17%. It is excreted unchanged in the urine with excretion of a single dose completed within 24 hours. Unabsorbed drug is excreted in the feces.Zanamivir is renally excreted as unchanged drug. 2.5 - 10.9 L/h [Following oral inhalation 10 mg] 5.3 L/h [Normal renal function receiving IV single dose of 4 mg or 2 mg] 2.7 L/h [Patients with mild and moderate renal impairement receiving IV single dose of 4 mg or 2 mg] 0.8 L/h [Patients with severe renal impairement receiving IV single dose of 4 mg or 2 mg] Protein binding: Very low (<10%). Orally inhaled zanamivir is systemically absorbed, approximately 4% to 17%. Elimination: Renal: Excreted unchanged in the urine with excretion of a single dose completed within 24 hours. Total clearance ranges from 2.5 to 10.9 L/hr. Fecal: Unabsorbed drug is secreted in the feces. Time to peak effect: 72 hours. For more Absorption, Distribution and Excretion (Complete) data for ZANAMIVIR (7 total), please visit the HSDB record page. Metabolism / Metabolites Not metabolized Not metabolized. Biological Half-Life 2.5-5.1 hours The serum half-life of zanamivir following administration by oral inhalation ranges from 2.5 to 5.1 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In randomized controlled trials, 2% to 3% of zanamivir recipients developed ALT or AST elevations above twice the upper limit of the normal range, but a similar rate was found in placebo-treated patients. Despite widespread use, there is little evidence that zanamivir when used by inhalation causes liver injury, either in the form of asymptomatic serum enzyme elevations or clinically apparent liver disease. In pilot studies of intravenous zanamivir for severe influenza, serum enzyme elevations have been reported in ~10% of patients, occasionally with jaundice, but the role of zanamivir versus the underlying severe viral infection has not been defined. Likelihood score: E (unlikely cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the use of zanamivir during breastfeeding. One group of authors estimated that an exclusively breastfed 5 kg infant would receive about 0.075 mg daily in breastmilk after an inhaled maternal dose of 10 mg, which is less than 1% of the dose in older children. In addition, because zanamivir is poorly absorbed orally, it is not likely to reach the bloodstream of the infant in clinically important amounts. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Zanamivir has limited plasma protein binding (<10%). Interactions Zanamivir is not a substrate nor does it affect cytochrome P450 (CYP) isoenzymes (CYP1A1/2, 2A6, 2C9, 2C18, 2D6, 2E1, and 3A4) in human liver microsomes. |
| 参考文献 |
|
| 其他信息 |
Zanamivir is a member of guanidines. It has a role as an EC 3.2.1.18 (exo-alpha-sialidase) inhibitor and an antiviral agent.
A guanido-neuraminic acid that is used to inhibit neuraminidase. Zanamivir is a Neuraminidase Inhibitor. The mechanism of action of zanamivir is as a Neuraminidase Inhibitor. Zanamivir is an inhibitor of the influenza neuraminidase enzyme and is given by inhalation as therapy and prophylaxis against influenza A and B. Zanamivir has not been associated with clinically apparent liver injury, at least when given by inhalation. Zanamivir is a sialic acid-analogue neuraminidase inhibitor with antiviral activity. Administered into the respiratory tract by aerosol inhalation, zanamivir selectively binds to and inhibits influenza A and B virus neuraminidase-mediated cleavage of sialic acid residues in host cell membrane-bound glycoprotein receptors for influenza viruses, preventing the release of progeny viruses from host cell surfaces and, so, further viral replication. A guanido-neuraminic acid that is used to inhibit NEURAMINIDASE. Drug Indication For the prevention and treatment of influenza A and B. FDA Label Dectova is indicated for the treatment of complicated and potentially life-threatening influenza A or B virus infection in adult and paediatric patients (aged â¥6 months) when: The patient's influenza virus is known or suspected to be resistant to anti-influenza medicinal products other than zanamivir, and/orOther anti-viral medicinal products for treatment of influenza, including inhaled zanamivir, are not suitable for the individual patient. Dectova should be used in accordance with official guidance. Prevention of influenza, Treatment of influenza Mechanism of Action The proposed mechanism of action of zanamivir is via inhibition of influenza virus neuraminidase with the possibility of alteration of virus particle aggregation and release. By binding and inhibiting the neuraminidase protein, the drug renders the influenza virus unable to escape its host cell and infect others. Zanamivir is a potent selective competitive inhibitor of the influenza virus neuraminidase, an enzyme essential for viral replication. Neuraminidase cleaves terminal sialic acid residues from glycoconjugates to enable the release of virus from infected cells, prevent the formation of viral aggragates after release from host cells, and possibly decrease viral inactivation by respiratory mucous. Zanamivir is a selective inhibitor of influenza A and B virus neuraminidase, possibly altering particle aggregation and release. Therapeutic Uses Antiviral Agents; Enzyme Inhibitors At this time, CDC recommends the use of oseltamivir or zanamivir for the treatment of infection with swine influenza (H1N1) viruses. MEDICATION: Antiviral; Influenza viral neuraminidase inhibitor Zanamivir is indicated for the treatment of uncomplicated acute illness due to influenza A virus in adults and children 7 years and older who have been symptomatic for no more than 2 days. Zanamivir must be started within 48 hours after the onset of influenza symptoms. /Included in US product labeling/ For more Therapeutic Uses (Complete) data for ZANAMIVIR (6 total), please visit the HSDB record page. Drug Warnings Swine influenza (H1N1) viruses contain a unique combination of gene segments that have not been reported previously among swine or human influenza viruses in the US or elsewhere. The H1N1 viruses are resistant to amantadine and rimantadine but not to oseltamivir or zanamivir. Bronchospasm and decline in lung function have been reported in some patients receiving relenza. Many but not all of these patients had underlying airways disease such as asthma or chronic obstructive pulmonary disease. Because of the risk of serious adverse events and because efficacy has not been demonstrated in this population, /zanamivir/ is not generally recommend for treatment of patients with underlying airways disease. Some patients with serious adverse events during treatment with /zanamivir/ have had fatal outcomes, although causality was difficult to assess. /Zanamivir/ should be discontinued in any patient who develops bronchospasm or decline in respratory function; immediate treatment and hospitalization may be required. Some patients without prior pulmonary disease may also have respiratory abnormalities from acute respiratory infection that could resemble adverse drug reactions or increase patient vulnerability to adverse drug reactions. FDA Pregnancy Risk Category: B /NO EVIDENCE OF RISK IN HUMANS. Adequate, well controlled studies in pregnant women have not shown increased risk of fetal abnormalities despite adverse findings in animals, or, in the absence of adequate human studies, animal studies show no fetal risk. The chance of fetal harm is remote but remains a possibility./ Adverse effects occurring in 1-3% or more of adults and children 12 years of age or older include diarrhea; nausea; vomiting; nasal signs and symptoms; bronchitis; sinusitis; cough; ear, nose, and throat infections; headache; and dizziness. No adverse effect occurred at an incidence greater than 3%. Adverse effects occurring in up to 5% of children 5-12 years of age include ear, nose, and throat infections; vomiting; nausea; and diarrhea. Some adverse effects may be secondary to lactose vehicle inhalation. Bronchospasm and allergic-like reactions, including oropharyngeal edema and serious rash, have been reported. Unlike amantadine and rimantadine, neuraminidase inhibitors like zanamivir do not appear to adversely affect the CNS. For more Drug Warnings (Complete) data for ZANAMIVIR (10 total), please visit the HSDB record page. Pharmacodynamics Zanamivir, an antiviral agent, is a neuraminidase inhibitor indicated for treatment of uncomplicated acute illness due to influenza A and B virus in adults and pediatric patients 7 years and older who have been symptomatic for no more than 2 days. Zanamivir has also been shown to significantly inhibit the human sialidases NEU3 and NEU2 in the micromolar range (Ki 3.7 +/-0.48 and 12.9+/-0.07 microM, respectively), which could account for some of the rare side effects of zanamivir. |
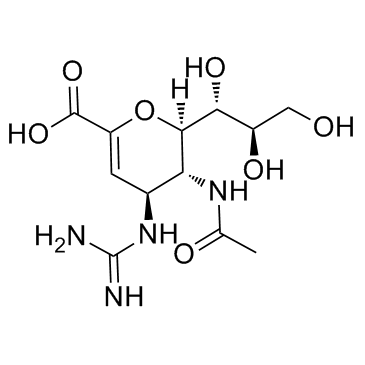
| 分子式 |
C12H20N4O7
|
|---|---|
| 分子量 |
332.313
|
| 精确质量 |
332.133
|
| 元素分析 |
C, 43.37; H, 6.07; N, 16.86; O, 33.70
|
| CAS号 |
139110-80-8
|
| 相关CAS号 |
Zanamivir (hydrate)(5:1);171094-50-1
|
| PubChem CID |
60855
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.8±0.1 g/cm3
|
| 熔点 |
256ºC (dec.)
|
| 折射率 |
1.679
|
| LogP |
-4.13
|
| tPSA |
198.22
|
| 氢键供体(HBD)数目 |
7
|
| 氢键受体(HBA)数目 |
8
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
23
|
| 分子复杂度/Complexity |
518
|
| 定义原子立体中心数目 |
5
|
| SMILES |
O1C(C(=O)O[H])=C([H])[C@@]([H])([C@]([H])([C@]1([H])[C@@]([H])([C@@]([H])(C([H])([H])O[H])O[H])O[H])N([H])C(C([H])([H])[H])=O)/N=C(\N([H])[H])/N([H])[H]
|
| InChi Key |
ARAIBEBZBOPLMB-UFGQHTETSA-N
|
| InChi Code |
InChI=1S/C12H20N4O7/c1-4(18)15-8-5(16-12(13)14)2-7(11(21)22)23-10(8)9(20)6(19)3-17/h2,5-6,8-10,17,19-20H,3H2,1H3,(H,15,18)(H,21,22)(H4,13,14,16)/t5-,6+,8+,9+,10+/m0/s1
|
| 化学名 |
(2R,3R,4S)-3-acetamido-4-(diaminomethylideneamino)-2-[(1R,2R)-1,2,3-trihydroxypropyl]-3,4-dihydro-2H-pyran-6-carboxylic acid
|
| 别名 |
Zanamivir; GR-121167X; GG167; GR121167X; GG-167; GR 121167X; GG 167; trade name Relenza.
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O : 33.33~36 mg/mL (~100.30 mM)
DMSO : ~66 mg/mL ( ~198.6 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 9.09 mg/mL (27.35 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。 (<60°C).
请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.0092 mL | 15.0462 mL | 30.0924 mL | |
| 5 mM | 0.6018 mL | 3.0092 mL | 6.0185 mL | |
| 10 mM | 0.3009 mL | 1.5046 mL | 3.0092 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。