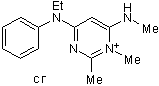
| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 25mg |
|
||
| 50mg |
|
||
| 100mg | |||
| 250mg | |||
| Other Sizes |
| 靶点 |
HCN/hyperpolarization-activated cyclic nucleotide-gated channel
|
|---|---|
| 体外研究 (In Vitro) |
ZD7288充当激活环标记门控 (HCN) 通道阻断剂,包括瞬态和超瞬态。 ZD7288以浓度抑制方式抑制谷胱甘肽释放。经过 1、5 和 50 μM ZD7288 24 小时缓冲期后,细胞外液的谷氨酸水平分别降至 69.0±2.8%、31.4±2.0% 和 4.4±0.3%(与对照组相比,P<0.01)。 DMEM/F12组比率[100.2±4.2%])。添加 ZD7288 (25) 个氨基酸 20 分钟后,50 μM 谷氨酸产生的 [Ca2+]i 分别增加至谷氨酸的 59.2±2.7%、41.4±2.3% 和 21.0±1.4%(P <0.01,与 50 μM 谷氨酸组比较)[1]。
接下来,我们研究了ZD7288对培养的海马神经元谷氨酸释放的影响。ZD7288以浓度依赖的方式抑制谷氨酸的释放(图3B)。用1、5和50µM ZD7288孵育24小时后,细胞外液中的谷氨酸含量分别降至69.0±2.8%、31.4±2.0%和4.4±0.3%(与DMEM/F12组[100.2±4.2%]相比,P<0.01)。 据报道,通过提高cAMP水平可以增强Ih通道的活性。为了证实ZD7288对谷氨酸释放的抑制作用是依赖于Ih通道的,我们使用毛喉素和8-Br-cAMP探索了cAMP水平的药理学升高对谷氨酸分泌的影响。用5和50µM 8-Br-cAMP孵育后,细胞外液中的谷氨酸含量分别增加到136.1±7.4%和188.1±13.8%(与DMEM/F12组相比,P<0.01)。此外,与毛喉素(1和5µM)一起孵育后,细胞外谷氨酸含量分别增加到177.6±6.8%和308.7±6.9%(与DMEM/F12组相比,P<0.01)。 ZD7288对谷氨酸诱导的大鼠海马神经元[Ca2+]i升高的影响[1] 细胞内钙在递质释放中起着重要作用。因此,我们还测量了ZD7288对细胞内钙水平的影响。在培养的海马神经元中,谷氨酸(50µM)引起[Ca2+]i显著增加,比基线增加78.0±3.3%。与ZD7288(25、50或100µM)孵育20分钟后,50µM谷氨酸诱导的[Ca2+]i升高分别减弱至59.2±2.7%、41.4±2.3%和21.0±1.4%(与50µM的谷氨酸组相比,P<0.01;图4)。ZD7288以浓度依赖的方式减弱谷氨酸诱导的[Ca2+]i升高。我们还探讨了8-Br-cAMP对谷氨酸诱导的[Ca2+]i升高的影响。8-Br-cAMP促进谷氨酸诱导的[Ca2+]i升高(图4)。与5和50µM 8-Br-cAMP孵育5分钟后,谷氨酸诱导的[Ca2+]i分别增加了101.3±3.1%和125.4±3.4%(与50µM谷氨酸组相比,P<0.01)。与ZD7288孵育20分钟后,50µM 8-Br-cAMP使谷氨酸诱导的[Ca2+]i升高了86.2±3.3%(与50µM 8-Br-cAMP组相比,P<0.01)。用50µM ZD7288处理几乎完全逆转了[Ca2+]i中8-Br-cAMP的促进作用。 |
| 体内研究 (In Vivo) |
在高频刺激前 5 分钟应用 ZD72880.1 μM 时,场兴奋性突触后电位 (fEPSP) 的幅度显着降低。这种抑制作用在记录期间持续存在。高频刺激 30 分钟后,应用 0.1 μM ZD7288 几乎逆转了已建立的长时程增强 (LTP)。高频刺激前 5 分钟,与生理盐水组相比,应用 ZD7288 (0.1 μM) 导致谷氨酸含量降低 74.9±8.0% (P<0.05)。此外,高频刺激30分钟后,应用0.1 μM ZD7288可显着降低谷氨酸浓度,与生理盐水组相比达到77.0%±9.4%(P<0.05)[1]。
ZD7288对大鼠执行通路CA3突触LTP诱导的影响[1] 我们之前的体内研究表明,ZD7288以浓度依赖的方式抑制了穿孔通路CA3突触的基础突触传递(Zheng等人,2006)。在这里,我们已经证明ZD7288和CsCl阻断了LTP在执行通路CA3突触的诱导。在90分钟的记录期内,高频刺激导致接受生理盐水的大鼠fEPSP振幅显著增加,fEPSP的平均幅度为基线值的281.8±6.6%(P<0.05;图1)。通过高频刺激穿孔通路纤维,在穿孔通路-CA3突触中诱导LTP。然而,在高频刺激前5分钟应用0.1µM的ZD7288显著降低了fEPSP的振幅,并且这种抑制作用在整个记录期间都保持不变。高频刺激后30、60和90分钟,fEPSP振幅分别为基线的92.4±10.1%、85.6±12.0%和85.2±11.8%(与生理盐水组相比,P<0.01)。ZD7288显著抑制了LTP的诱导。 为了证实ZD7288诱导的LTP减少是由于其阻断Ih通道的作用,我们使用了另一种已知的Ih阻断剂Cs+,通常用作Ih的诊断工具(Wickenden等人,2009),以测试其对LTP诱导的影响。Cs+(1 mM)显著抑制了LTP在执行通路CA3合成酶的诱导。在高频刺激前5分钟施加CsCl(1 mM)后,fEPSP振幅在每个时间点都显著降低。高频刺激后30、60和90分钟,fEPSP振幅分别为基线的41.6±12.8%、75.6±11.6%和78.1±5.5%(与生理盐水组相比,P<0.01)。Cs+对LTP的抑制作用随时间减弱。我们的结果表明,Ih通道参与了执行通路-CA3突触LTP的诱导。 ZD7288对大鼠执行通路CA3突触LTP维持的影响[1] 为了进一步研究Ih通道在LTP维持中的作用,研究了ZD7288对先前建立的LTP的影响。高频刺激30分钟后应用0.1µM ZD7288几乎完全逆转了已建立的LTP(图2)。高频刺激后60分钟和90分钟,振幅分别为基线的92.6±6.4%和88.9±7.6%(与生理盐水组相比,P<0.01)。高频刺激30分钟后应用1 mM CsCl产生了类似的抑制作用。高频刺激后60分钟和90分钟,fEPSP振幅分别为基线的62.6±7.6%和84.8±18.8%(与生理盐水组相比,P<0.01)。此外,CsCl的抑制作用随时间而降低。这些结果表明,ZD7288和Cs+阻断了大鼠执行通路-CA3突触的LTP维持。 ZD7288对海马谷氨酸释放的影响[1] 在一些神经元中,突触前Ih通道通过控制递质释放来调节突触传递。谷氨酸在执行通路CA3突触的LTP形成中起着重要作用,其中LTP诱导依赖于N-甲基-D-天冬氨酸受体(McMahon和Barrionuevo,2002)。我们研究了ZD7288对海马组织谷氨酸释放的影响。高频刺激的应用导致接受生理盐水的大鼠谷氨酸水平略有升高(图3A)。谷氨酸水平增加到111.1±9.6%(与接受生理盐水和无高频刺激的对照组大鼠相比,P>0.05[138.4±34.3µmol/g蛋白质,标准化为100±8.8%])。在高频刺激前5分钟应用ZD7288(0.1µM)后,谷氨酸含量降至74.9±8.0%(与生理盐水组相比,P<0.05)。在高频刺激前施加CsCl(1mM)产生了与ZD7288相同的效果;谷氨酸含量降至71.9±10.0%(与生理盐水组相比,P<0.05)。此外,在高频刺激30分钟后应用0.1µM ZD7288,谷氨酸含量显著降低至77.0%±9.4%(与生理盐水组相比,P<0.05)。高频刺激后用1mM CsCl处理,谷氨酸含量降至82.5%±9.1%。然而,与生理盐水组相比,没有显著差异(P>0.05)。 |
| 细胞实验 |
神经元培养[1]
从新生(1-2天大)Sprague-Dawley大鼠中获得原代海马神经元。将大鼠斩首,迅速取出大脑,放入冰冷的磷酸盐缓冲盐水中。将海马体解剖出来,在37°C下用0.125%胰蛋白酶消化20分钟,然后进行机械分离和1000×g离心8分钟。将细胞重新悬浮并铺在96孔板(用于氨基酸分析)或聚-D-赖氨酸涂层盖玻片(用于测量内部钙浓度[Ca2+]i)上。神经元在含有10%胎牛血清、100 U/L青霉素、100 mg/L链霉素和0.5 mM谷氨酰胺的Dulbecco改良Eagle培养基/Ham营养混合物F12中培养,并在5%CO2培养箱中保持在37°C。在72小时时向培养基中加入10mg/L阿拉伯糖基胞嘧啶以减少非神经元细胞的数量。每两天更换一半的培养基。实验在第8-11天进行。将细胞与ZD7288(1、5或50µM)、8-溴腺苷环腺苷酸(8-Br-cAMP,5或50μM)或毛喉素(1或5µM)一起孵育24小时,收集培养基进行谷氨酸测定。 谷氨酸测量[1] LTP记录后,小心地将同侧海马体与大脑分离,用冰冷的生理盐水清洗,并在-80°C下冷冻直至处理。将解冻的组织在0.4M高氯酸中均质化。取等分试样进行蛋白质测定后,将匀浆在4°C下以10000×g离心15分钟。然后用2M KHCO3中和匀浆,并在3000×g下离心5分钟。上清液在-80°C下冷冻进行分析。使用考马斯亮蓝测量蛋白质水平。药物孵育24小时后收集神经元培养基,在3000×g下离心5分钟。上清液储存在-80°C下进行分析。如前所述(McMahon和Barrionuevo,2002),在用O-邻苯二甲醛 进行自动柱前衍生化后,使用高效液相色谱法(Kromasil ODS2 C18柱)进行谷氨酸分析,并使用分光光度计进行荧光检测(激发波长330 nm,发射波长420 nm)。根据每个峰面积,使用外标法定量谷氨酸的浓度。数据根据从输注生理盐水的海马组织和用DMEM/F12处理的神经元中获得的基线谷氨酸值进行归一化。每个样品中的谷氨酸水平表示为样品中谷氨酸与基线值的比率。 [Ca2+]i[1]的测量 [Ca2+]i测量是根据我们之前在神经元中的研究进行的(Huang等人,2009)。将培养的海马神经元与1µM Fura-2乙酰氧基羟甲基酯在37°C下孵育30分钟,用人工脑脊液(含有140 mM NaCl、5 mM KCl、2 mM CaCl2、1 mM MgCl2、10 mM葡萄糖和10 mM羟乙基哌嗪乙磺酸,pH 7.3)洗涤三次,然后在室温下在黑暗中孵育30 min。通过Ratio Vision数字荧光显微镜系统观察到Fura-2荧光。荧光信号由340和380nm激发波长诱发,并由TILLvisION 4.0软件在510nm处收集。340:380 nm荧光比用于表示[Ca2+]i。峰值钙变化表示为从基线增加的百分比。在用50µM谷氨酸刺激之前,神经元在ZD7288(25、50或100µM)或8-Br-cAMP(5或50µM)中孵育15分钟。所有实验重复三次,在4-5个培养皿中使用不同批次的细胞。 |
| 动物实验 |
Electrophysiological recordings in vivo [1]
Animals were anesthetized intraperitoneally with urethane 1.2 g/kg and fixed in a stereotaxic frame. Body temperature was kept at 37 ± 0.5°C during the experiment, using a constant temperature water cycling system. The skull landmark bregma was chosen as the stereotaxic reference point. Small holes were made in the skull and a stimulating electrode (bipolar stainless steel, 140 µm diameter) was placed at the perforant path (6.8–7.0 mm anteroposterior, 4.3–4.5 mm rostrolateral, depth 3.0–4.0 mm) and a recording electrode (monopolar stainless steel, 140 µm diameter) was positioned in ipsilateral hippocampal CA3 (3.3–3.5 mm anteroposterior, 3.3–3.5 mm rostrolateral). The depth of the recording electrode was determined by the maximal response. Test stimuli were given every 2 seconds (0.5 Hz, 0.15 ms duration) with a programmable electric stimulator using an isolation unit. Field excitatory postsynaptic potentials (fEPSPs) were acquired, amplified, monitored and analyzed with a SMUP-PC biology signal processing system. Baseline fEPSPs were recorded at 50–60% of the maximal response. LTP was then induced by a series of high-frequency stimuli (4 trains of 50 pulses at 100 Hz, 150 µs duration, intertrain interval of 20 seconds). fEPSPs were recorded 90 minutes after high-frequency stimulation. Baseline values were calculated by taking the mean EPSP amplitude at 5 different time points within 30 minutes before high-frequency stimulation. The ratio of absolute fEPSP amplitude to baseline value was used to describe the amplitude level. For hippocampal administration of saline or drugs, a cannula was carefully inserted into the CA3 area with an introductory tube fixed parallel to the recording electrode, reaching 0.1–0.2 mm higher than the electrode tip. To test the effects of blockers on the induction of LTP, we applied 0.1 µM ZD7288 or the monovalent cation cesium (Cs+), a known nonspecific Ih antagonist (5 µM CsCl) 5 minutes before high-frequency stimulation. To test the effects of blockers on the maintenance of LTP, ZD7288/Cs+ was slowly administered using an infusion/withdrawal pump 30 minutes after the high-frequency stimulation. |
| 参考文献 | |
| 其他信息 |
4-(N-Ethyl-N-phenylamino)-1,2-dimethyl-6-(methylamino)pyrimidinium chloride is an organic molecular entity.
The selective hyperpolarization-activated cyclic nucleotide-gated (HCN) channel blocker 4-(N-ethyl-N-phenylamino)-1,2-dimethyl-6-(methylamino) pyrimidinium chloride (ZD7288) blocks the induction of long-term potentiation in the perforant path-CA3 region in rat hippocampus in vivo. To explore the mechanisms underlying the action of ZD7288, we recorded excitatory postsynaptic potentials in perforant path-CA3 synapses in male Sprague-Dawley rats. We measured glutamate content in the hippocampus and in cultured hippocampal neurons using high performance liquid chromatography, and determined intracellular Ca(2+) concentration [Ca(2+)]i) using Fura-2. ZD7288 inhibited the induction and maintenance of long-term potentiation, and these effects were mirrored by the nonspecific HCN channel blocker cesium. ZD7288 also decreased glutamate release in hippocampal tissue and in cultured hippocampal neurons. Furthermore, ZD7288 attenuated glutamate-induced rises in [Ca(2+)]i in a concentration-dependent manner and reversed 8-Br-cAMP-mediated facilitation of these glutamate-induced [Ca(2+)]i rises. Our results suggest that ZD7288 inhibits hippocampal synaptic plasticity both glutamate release and resultant [Ca(2+)]i increases in rat hippocampal neurons.[1] Ih channels play important roles in regulating excitability and rhythmic activity of neurons. Ih channels are also important in synaptic modulation and plasticity. In a well-known trisynaptic model of hippocampal circuitry, ZD7288 depressed synaptic transmission at perforant path–granule cell synapses by inhibiting postsynaptic glutamate receptors (Chen, 2004). ZD7288-induced reduction of mossy fiber LTP is due to its inhibition of neurotransmitter release (Mellor et al., 2002). Our previous study demonstrated that Ih channels were also involved in Schaffer–CA1 pathway LTP via inhibiting N-methyl-D-aspartate receptor function (He et al., 2010). Here, we investigated the role of Ih in synaptic plasticity of the direct cortical projection to the hippocampus. We focused on the perforant path–CA3 synapse, the major route of cortical projection to the hippocampal CA3 area, which mediates memory retrieval. In agreement with previous studies indicating that perforant path inputs might be capable of driving CA3 pyramidal cells to fire (Urban et al., 1998; McMahon and Barrionuevo, 2002), our data demonstrated that perforant path fiber stimulation induces LTP, the average fEPSP amplitude being sustained at 282% of baseline for over 1 hour. Furthermore, treatment with ZD7288 inhibited LTP induction and completely reversed the established LTP, and the inhibitory effects were maintained for at least 1 hour. [1] In addition to blocking Ih channels, which may result in nonspecific inhibition of the postsynaptic glutamate receptor, ZD7288 depresses LTP by inhibition of postsynaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid and N-methyl-D-aspartate receptor-mediated responses at the perforant path–granule cell synapse (Chen, 2004). McMahon and Barrionuevo (2002) showed that perforant path–CA3 LTP is N-methyl-D-aspartate receptor-dependent. To investigate whether ZD7288-induced synaptic depression is a specific consequence of Ih channel blockade, we investigated whether its effects were similar to another Ih channel blocker, CsCl. Indeed, 1 mM CsCl produced a comparable LTP-inhibiting effect to ZD7288, blocking LTP induction when applied before high-frequency stimulation, and reversing it when applied after high-frequency stimulation. These data demonstrate that the induction and maintenance of LTP are both suppressed when Ih channels are blocked, suggesting that Ih channels might directly participate in the process of induction and maintenance of LTP. However, the inhibitory effect of CsCl gradually attenuated over time. As Cs+ is a nonselective Ih channel blocker, inhibition of potassium channels may have contributed to this attenuation. [1] Ih channels are widely distributed in the nervous system, and have been identified in mammalian presynaptic terminals (Southan et al., 2000; Cuttle et al., 2001). The channels are assembled as HCN1–HCN4 subunits. HCN1 and HCN2 are presynaptic and localized in the CA3 pyramidal cell layer (Notomi and Shigemoto, 2004). Many previous studies have revealed that functional presynaptic Ih channels play significant roles in synaptic transmission and long-term plasticity by controlling transmitter release (Beaumont and Zucker, 2000; Mellor et al., 2002; Huang and Hsu, 2003). To explore the possibility that ZD7288 depressed perforant path–CA3 pathway synaptic plasticity via a presynaptic mechanism, we examined the effects of ZD7288 on glutamate release. We found that treated with ZD7288 before and after high-frequency stimulation the glutamate content of hippocampal cells was lower than that of the saline group, and CsCl (1 mM) also inhibited glutamate release. The increase in the level of glutamate after high-frequency stimulation indicated that the stimulation activated glutamatergic neurons. However, the glutamate increase was not significantly different from control. It is probably because that the whole hippocampus was used in this study to detect glutamate content. Therefore, we further studied the effects of ZD7288 on glutamate release using cultured hippocampal neurons. As predicted, ZD7288 markedly inhibited glutamate release in the cultured cells. Ih channels are activated not only by hyperpolarization, but also by the gating of intracellular cAMP levels. cAMP enhances the activity of Ih channels by directly binding to the channel or by indirect activation of protein kinase A (Lüthi and McCormick, 1998; Abi-Gerges et al., 2000; Mellor et al., 2002; Genlain et al., 2007). Increased intracellular cAMP and activation of protein kinase A are essential for the generation of LTP (Pape, 1996; Mellor et al., 2002). ZD7288 inhibits cAMP-triggered increases of miniature excitatory postsynaptic current frequency by blockade of Ih channels (Genlain et al., 2007). Here, cAMP level was elevated by applying the cAMP analog 8-Br-cAMP and the adenylyl-cyclase activator forskolin. We found that both 8-Br-cAMP and forskolin increased glutamate release in cultured hippocampal neurons. These results suggest that Ih channels are involved in glutamate release. ZD7288 inhibited LTP formation by depressing glutamate release and by blocking Ih channels. [1] Two mechanisms have been proposed to underlie Ih channel modulation of glutamate release. One is associated with Ca2+-induced Ca2+ release from the store and suggests that the activation of Ih channels accompanied by Ca2+ influx triggers the process of modulation (Yu et al., 2004). The other proposed mechanism is that Ih channels directly couple to the release machinery in a calcium-independent manner (Beaumont and Zucker, 2000). In the present study, to test whether the ZD7288-induced inhibition of glutamate release was associated with intracellular calcium, glutamate was used as an activator to induce a rise in [Ca2+]i. ZD7288 inhibited glutamate-induced [Ca2+]i increases in a concentration-dependent manner and reversed the 8-Br-cAMP-evoked rise in [Ca2+]i. Our data suggest that the inhibitory effect of ZD7288 on glutamate release results from its attenuation of [Ca2+]i. The activation of Ih channels may enhance Ca2+ influx into the presynaptic terminal by depolarization; the opening of voltage-dependent calcium channels would, in turn, lead to persistent enhancement of glutamate release. More research is needed to explore the details of this proposed mechanism. [1] Ih channels are involved in many diseases, including epilepsy, vascular dementia and peripheral neuralgia (Li et al., 2010; Takasu et al., 2010; DiFrancesco et al., 2011). Ischemia is one of the commonest damaging factors in the nervous system. The excessive release of glutamate and the overload of intracellular Ca2+ play key roles in ischemic neuronal death (Mori et al., 2004; Zhao et al., 2006). Neuronal hyperexcitability enhances calcium influx, which subsequently triggers the release of excitatory neurotransmitters, especially glutamate. The excessive release of glutamate can lead to extra Ca2+ influx during ischemia. Ih channels are involved in ischemic lesions. Our previous studies showed that HCN1 mRNA and protein were decreased in chronic incomplete global cerebral ischemia (Li et al., 2010). We propose that the Ih channel blocker ZD7288 inhibits both glutamate release and the glutamate-induced rise in [Ca2+]i, which might contribute to its neuroprotective effects under conditions of cerebral ischemia. [1] In conclusion, the Ih channel blocker ZD7288 can markedly suppress LTP at perforant path–CA3 synapses. The inhibitory effect is likely due to attenuating release of glutamate and glutamate-induced [Ca2+]i rise in rat hippocampal neurons. |
| 分子式 |
C15H21CLN4
|
|---|---|
| 分子量 |
292.807041883469
|
| 精确质量 |
292.145
|
| 元素分析 |
C, 70.28; H, 7.86; N, 21.86
|
| CAS号 |
133059-99-1
|
| PubChem CID |
123983
|
| 外观&性状 |
White to off-white solid powder
|
| 沸点 |
359.9ºC at 760 mmHg
|
| 闪点 |
171.4ºC
|
| 蒸汽压 |
2.31E-05mmHg at 25°C
|
| tPSA |
32.04
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
20
|
| 分子复杂度/Complexity |
402
|
| 定义原子立体中心数目 |
0
|
| SMILES |
Cl.N(C1C=CC=CC=1)(CC)C1=C/C(=N\C)/N(C)C(C)=N1
|
| InChi Key |
DUWKUHWHTPRMAP-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C15H20N4.ClH/c1-5-19(13-9-7-6-8-10-13)15-11-14(16-3)18(4)12(2)17-15;/h6-11H,5H2,1-4H3;1H
|
| 化学名 |
N-ethyl-1,2-dimethyl-6-methylimino-N-phenylpyrimidin-4-amine;hydrochloride
|
| 别名 |
ZD 7288; ZD-7288; 133059-99-1; 4-Pyrimidinamine, N-ethyl-1,6-dihydro-1,2-dimethyl-6-(methylimino)-N-phenyl-, hydrochloride (1:1); 4-(N-Ethyl-N-phenylamino)-1,2-dimethyl-6-(methylamino)pyrimidinium chloride; Zeneca ZD7288; N-ethyl-1,2-dimethyl-6-methylimino-N-phenylpyrimidin-4-amine;hydrochloride; 4-Pyrimidinamine, N-ethyl-1,6-dihydro-1,2-dimethyl-6-(methylimino)-N-phenyl-, monohydrochloride; Ici-D2788; ZD7288
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~50 mg/mL (~170.76 mM)
H2O : ≥ 50 mg/mL (~170.76 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 100 mg/mL (341.52 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。
请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.4152 mL | 17.0759 mL | 34.1518 mL | |
| 5 mM | 0.6830 mL | 3.4152 mL | 6.8304 mL | |
| 10 mM | 0.3415 mL | 1.7076 mL | 3.4152 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。