| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 靶点 |
|
|
|---|---|---|
| 体外研究 (In Vitro) |
4-羟基壬烯醛除了是 ALDH2 的抑制剂外,也是 ALDH2 的底物;在低浓度下,4-羟基壬烯醛对 ALDH2 的抑制是可逆的,但在 10 μM 以上则变得不可逆。 4-为了控制自身合成并提高细胞对氧化应激的防御能力,4-羟基壬烯醛可以触发抗氧化防御机制[1]。 4-脂质过氧化的副产物 4-羟基壬烯醛对细菌、病毒和哺乳动物细胞具有遗传毒性和致突变性。所有四种 DNA 碱基都会发生反应,但效率不同:G > C > A > T。4-Hydroxynonenal 基因毒性作用最可靠的生物标志物是 4-Hydroxynonenal-dG,这些加合物主要在细胞核 DNA 中鉴定。 4-羟基壬烯醛-dG 引起的 p53 突变是 4-羟基壬烯醛-dG 在人类恶性肿瘤中病因学意义的众所周知的例证。 4-羟基壬烯醛-dG 加合物优先在 p53 基因密码子 249 的第三个碱基处形成。这导致基因突变并改变了许多生物过程,例如分化、细胞凋亡、细胞周期停滞和 DNA 修复[1]。
|
|
| 体内研究 (In Vivo) |
流体冲击损伤 (FPI) 后 24 小时,测量小鼠脑组织中 NADPH 氧化酶 1 (NOX1)、诱导型一氧化氮合酶 (iNOS) 和 4-羟基壬烯醛 (4-HNE) 的表达水平。与未受伤的 Nrf2+/+ 和 Nrf2-/- 小鼠相比,野生型 (Nrf2+/+) 和 Nrf2 缺陷型 (Nrf2-/-) 小鼠在 15 psi 损伤(中度损伤)后表现出 4-Hydroxynonenal 表达增加。将 Nrf2-/-KO 小鼠与相应损伤和未损伤的 Nrf2+/+ WT 动物进行比较,这些动物中 4-羟基壬烯醛的表达水平要高得多,与 iNOS 结果一致[2]。
|
|
| 细胞实验 |
我们的初步工作表明,维生素D受体(VDR)的激活对顺铂诱导的急性肾损伤(AKI)具有保护作用。最近有报道称,铁下垂与AKI有关。在本研究中,我们研究了脱铁性贫血与VDR在顺铂诱导的AKI中的保护作用之间的内在关系。通过在体内和体外顺铂诱导的AKI模型中使用脱铁抑制剂ferrostatin-1并测量脱铁细胞死亡表型,我们观察到Ferrostatin1降低了血尿素氮、肌酐和组织损伤,从而验证了脱铁在顺铂诱导的AKI中的重要作用。VDR激动剂帕钙化醇可以通过降低脂质过氧化(脱铁症的特征表型)、生物标志物4-羟基壬烯醛(4HNE)和丙二醛(MDA),同时逆转谷胱甘肽过氧化物酶4(GPX4,脱铁症关键调节因子)的下调,在功能和组织学上减弱顺铂诱导的AKI。VDR敲除小鼠表现出比野生型小鼠更多的脱铁细胞死亡和加重肾损伤。在体内外顺铂胁迫下,VDR缺乏显著降低了GPX4的表达,进一步的荧光素酶报告基因分析表明GPX4是转录因子VDR的靶基因。此外,体外研究表明,siRNA对GPX4的抑制在很大程度上消除了帕钙醇对顺铂诱导的肾小管细胞损伤的保护作用。此外,帕钙醇预处理还可以减轻Erastin(脱铁诱导剂)诱导的HK-2细胞死亡。这些数据表明脱铁性贫血在顺铂诱导的AKI中起着重要作用。VDR激活可以通过部分通过GPX4的反式调节抑制脱铁性贫血来保护顺铂诱导的肾损伤[3]。
|
|
| 动物实验 |
Increased methylglyoxal (MG) formation is associated with diabetes and its complications. In zebrafish, knockout of the main MG detoxifying system Glyoxalase 1, led to limited MG elevation but significantly elevated aldehyde dehydrogenases (ALDH) activity and aldh3a1 expression, suggesting the compensatory role of Aldh3a1 in diabetes. To evaluate the function of Aldh3a1 in glucose homeostasis and diabetes, aldh3a1-/- zebrafish mutants were generated using CRISPR-Cas9. Vasculature and pancreas morphology were analysed by zebrafish transgenic reporter lines. Corresponding reactive carbonyl species (RCS), glucose, transcriptome and metabolomics screenings were performed and ALDH activity was measured for further verification. Aldh3a1-/- zebrafish larvae displayed retinal vasodilatory alterations, impaired glucose homeostasis, which can be aggravated via pdx1 silencing induced hyperglycaemia. Unexpectedly, MG was not altered, but 4-hydroxynonenal (4-HNE), another prominent lipid peroxidation RCS exhibited high affinity with Aldh3a1, was increased in aldh3a1 mutants. 4-HNE was responsible for the retinal phenotype via pancreas disruption induced hyperglycaemia and can be rescued via l-Carnosine treatment. Furthermore, in type 2 diabetic patients, serum 4-HNE was increased and correlated with disease progression. Thus, our data suggest impaired 4-HNE detoxification and elevated 4-HNE concentration as biomarkers but also the possible inducers for diabetes, from genetic susceptibility to the pathological progression[4].
|
|
| 药代性质 (ADME/PK) |
Metabolism / Metabolites
Uremic toxins tend to accumulate in the blood either through dietary excess or through poor filtration by the kidneys. Most uremic toxins are metabolic waste products and are normally excreted in the urine or feces. |
|
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Uremic toxins such as 4-Hydroxynonenal are actively transported into the kidneys via organic ion transporters (especially OAT3). Increased levels of uremic toxins can stimulate the production of reactive oxygen species. This seems to be mediated by the direct binding or inhibition by uremic toxins of the enzyme NADPH oxidase (especially NOX4 which is abundant in the kidneys and heart) (A7868). Reactive oxygen species can induce several different DNA methyltransferases (DNMTs) which are involved in the silencing of a protein known as KLOTHO. KLOTHO has been identified as having important roles in anti-aging, mineral metabolism, and vitamin D metabolism. A number of studies have indicated that KLOTHO mRNA and protein levels are reduced during acute or chronic kidney diseases in response to high local levels of reactive oxygen species (A7869). |
|
| 参考文献 |
[1]. Zhong H, et al. Role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: focusing on mitochondria. Redox Biol. 2015;4:193-9.
[2]. Csala M, et al. On the role of 4-hydroxynonenal in health and disease. Biochim Biophys Acta. 2015 May;1852(5):826-38. [3]. Bhowmick S, et al. Traumatic brain injury-induced downregulation of Nrf2 activates inflammatory response and apoptotic cell death. J Mol Med (Berl). 2019 Nov 22. [3]. Cell Death Dis. 2020 Jan 29;11(1):73. doi: 10.1038/s41419-020-2256-z. [4]. Redox Biol. 2020 Oct;37:101723. doi: 10.1016/j.redox.2020.101723. |
|
| 其他信息 |
4-hydroxynon-2-enal is an enal consisting of non-2-ene having an oxo group at the 1-position and a hydroxy group at the 4-position. It has a role as a human metabolite. It is a hydroxyaldehyde, an enal and a 4-hydroxynonenal.
4-Hydroxynonenal is a uremic toxin. Uremic toxins can be subdivided into three major groups based upon their chemical and physical characteristics: 1) small, water-soluble, non-protein-bound compounds, such as urea; 2) small, lipid-soluble and/or protein-bound compounds, such as the phenols and 3) larger so-called middle-molecules, such as beta2-microglobulin. Chronic exposure of uremic toxins can lead to a number of conditions including renal damage, chronic kidney disease and cardiovascular disease. 4-Hydroxynonenal (HNE), one of the major end products of lipid peroxidation, has been shown to be involved in signal transduction and available evidence suggests that it can affect cell cycle events in a concentration-dependent manner. glutathione S-transferases (GSTs) can modulate the intracellular concentrations of HNE by affecting its generation during lipid peroxidation by reducing hydroperoxides and also by converting it into a glutathione conjugate. Overexpression of the Alpha class GSTs in cells leads to lower steady-state levels of HNE, and these cells acquire resistance to apoptosis induced by lipid peroxidation-causing agents such as H(2)O(2), UVA, superoxide anion, and pro-oxidant xenobiotics, suggesting that signaling for apoptosis by these agents is transduced through HNE. Cells with the capacity to exclude HNE from the intracellular environment at a faster rate are relatively more resistant to apoptosis caused by H(2)O(2), UVA, superoxide anion, and pro-oxidant xenobiotics as well as by HNE, suggesting that HNE may be a common denominator in mechanisms of apoptosis caused by oxidative stress. Transfection of adherent cells with HNE-metabolizing GSTs leads to transformation of these cells due to depletion of HNE. (A3295). |
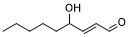
| 分子式 |
C₉H₁₆O₂
|
|---|---|
| 分子量 |
156.22
|
| 精确质量 |
156.115
|
| 元素分析 |
C, 69.19; H, 10.32; O, 20.48
|
| CAS号 |
75899-68-2
|
| 相关CAS号 |
4-Hydroxynonenal-d3;148706-06-3
|
| PubChem CID |
5283344
|
| 外观&性状 |
Colorless to light yellow liquid
|
| 密度 |
0.9±0.1 g/cm3
|
| 沸点 |
275.6±23.0 °C at 760 mmHg
|
| 闪点 |
115.2±15.2 °C
|
| 蒸汽压 |
0.0±1.3 mmHg at 25°C
|
| 折射率 |
1.460
|
| LogP |
1.85
|
| tPSA |
37.3
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
2
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
11
|
| 分子复杂度/Complexity |
119
|
| 定义原子立体中心数目 |
0
|
| SMILES |
CCCCCC(/C=C/C=O)O
|
| InChi Key |
JVJFIQYAHPMBBX-FNORWQNLSA-N
|
| InChi Code |
InChI=1S/C9H16O2/c1-2-3-4-6-9(11)7-5-8-10/h5,7-9,11H,2-4,6H2,1H3/b7-5+
|
| 化学名 |
4-hydroxy-2E-nonenal
|
| 别名 |
4 Hydroxynonenal; HNE;4-Hydroxynonenal; 4-Hydroxy-2-nonenal; 75899-68-2; 4-HNE; 4-hydroxynon-2-enal; (E)-4-hydroxynon-2-enal; trans-4-Hydroxy-2-nonenal; 4-Hydroxy-2,3-nonenal;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮和光照。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~640.12 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (16.00 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (13.31 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清的DMSO储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (13.31 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 6.4012 mL | 32.0061 mL | 64.0123 mL | |
| 5 mM | 1.2802 mL | 6.4012 mL | 12.8025 mL | |
| 10 mM | 0.6401 mL | 3.2006 mL | 6.4012 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。