| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|

| 靶点 |
Endogenous Metabolite
|
|---|---|
| 体外研究 (In Vitro) |
5-氨基乙酰丙酸 (5-ALA) 上调与防御和免疫相关的基因,改善有氧能量代谢,并增强南美白对虾对副溶血弧菌的免疫反应 [1]。
|
| 体内研究 (In Vivo) |
随着对虾养殖中几种传染病的出现,人们对使用饲料添加剂增强对虾免疫力的兴趣日益浓厚。最近,在血红素生物合成中起限速作用的非蛋白质氨基酸5-氨基酮戊酸(5-ALA)的使用因其对家畜免疫的积极作用而受到关注。为了评估5-ALA在南美白对虾(Litopenaeus vannamei)中的作用,我们进行了微阵列分析、副溶血弧菌浸泡激发试验、ATP水平测定以及与血红素合成和降解相关的一些血红蛋白和基因的基因表达分析。在微阵列上15745个南美白对虾推定基因中,101个基因在5-ALA补充组和对照组对虾肝胰腺之间差异表达超过四倍(p<0.05)。5-ALA上调了101个基因中的99个,其中41个是基于序列同源性的免疫和防御相关基因。与对照组相比,补充5-ALA的组在挑战试验中的存活率更高,胆色素原合酶、亚铁螯合酶、过氧化氢酶、核受体E75和血红素加氧酶-1的转录水平更高,ATP水平更高。这些发现表明,饮食中的5-ALA分别增强了凡纳对虾对副溶血弧菌的免疫反应,上调了免疫和防御相关基因,并增强了有氧能量代谢。需要进一步的研究来阐明5-ALA在虾养殖中的使用程度[1]。
|
| 酶活实验 |
检测97例ESCC患者病理标本中GPX4和HMOX1的表达,并进行预后分析。实时聚合酶链式反应(RT-PCR)、RNA微阵列和蛋白质印迹分析用于评估5-ALA在体外铁下垂中的作用。Ann Surg Oncol. 2021 Jul;28(7):3996-4006. https://pubmed.ncbi.nlm.nih.gov/33210267/
|
| 动物实验 |
Tumor volumetry was performed immediately prior to surgery. Tumor resection was then performed using the 5-ALA signal alone with the absence of a visible signal defining completeness of resection. This determination was carried out by the primary surgeon at all times. Functional neuronavigation data was intermittently projected to prevent inadvertent damage to functional brain areas. At the end of each stage of resection, the tumor cavity was systematically inspected to exclude residual tumor. Once the 5-ALA signal was undetectable, an iMRI scan was performed. If the extent of resection was confirmed, the decision to conclude the surgery was taken by the primary surgeon. Otherwise, the residual tumor volume was re-segmented and resection continued according to the neuronavigation. In all such cases the 5-ALA signal was redetected during further surgery once either the thin intervening layer of “healthy” brain parenchyma was removed and/or the viewing angle subsequently optimized. This procedure was repeated until the 5-ALA signal was no longer detectable, and the corresponding absence of contrast-enhancing tumor corroborated by iMRI. The additionally resected tissue detected by the iMRI was also analyzed by an experienced neuropathologist, confirming pathological glioma cell infiltration. In the event of persistence of 5-ALA in areas shown to be functional by the neuronavigation data, further surgery in the corresponding direction was intentionally terminated. PLoS One, 2012. 7(9): p. e44885.
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Oral bioavailability is 50-60%. ### **Topical gel** Pharmacokinetics (PK) of aminolevulinic acid (ALA) and PpIX was evaluated in a trial of 12 adult subjects with mild to moderate AK with at least 10 AK lesions on the face or forehead. A single dose of one entire tube of ALA (2 grams) was applied under occlusion for 3 hours followed by photodynamic therapy (PDT) to a total area of 20 cm2. The mean ± SD baseline plasma ALA and PpIX concentrations were 20.16 ± 16.53 ng/mL and 3.27 ± 2.40 ng/mL, respectively. In most subjects, an up to 2.5-fold increase of ALA plasma concentrations was observed during the first 3 hours after ALA application. The mean ± SD area under the concentration time curve (AUC0-t) and maximum concentration (Cmax) for baseline corrected ALA (n=12) were 142.83 ± 75.50 ng.h/mL and 27.19 ± 20.02 ng/mL, respectively. The median Tmax (time at which Cmax occurred) was 3 hours. ### **Topical solution** Two human pharmacokinetic (PK) studies were conducted in subjects with minimally to moderately thick actinic keratoses on the upper extremities, having at least 6 lesions on one upper extremity and at least 12 lesions on the other upper extremity. A single dose comprising of two topical applications of ALA topical solution (each containing 354 mg ALA HCl) were directly applied to the lesions and occluded for 3 hours prior to light treatment. The first PK study was conducted in 29 subjects and PK parameters of ALA were assessed. The baseline corrected mean ± SD of the maximum concentration (Cmax) of ALA was 249.9 ± 694.5 ng/mL and the median Tmax was 2 hours post dose. The mean ± SD exposure to ALA, as expressed by area under the concentration time curve (AUCt) was 669.9 ± 1610 ng·hr/mL. The mean ± SD elimination half-life (t1/2) of ALA was 5.7 ± 3.9 hours. A second PK study was conducted in 14 subjects and PK parameters of ALA and PpIX were measured. The baseline corrected PpIX concentrations were negative in at least 50% of samples in 50% (7/14) subjects and AUC could not be estimated reliably. The baseline-corrected mean ± SD of Cmax for ALA and PpIX was 95.6 ± 120.6 ng/mL and 0.95 ± 0.71 ng/mL, respectively. The median Tmax of ALA and PpIX was 2 hours post dose and 12 hours post dose, respectively. The mean AUCt of ALA was 261.1 ± 229.3 ng·hr/mL. The mean ± SD t1/2 of ALA was 8.5 ± 6.7 hours. ### **Oral solution** In 12 healthy subjects, the absolute bioavailability of ALA following the recommended dose of ALA solution was 100.0% + 1.1 with a range of 78.5% to 131.2%. Maximum ALA plasma concentrations were reached with a median of 0.8 hour (range 0.5 – 1.0 hour). In 12 healthy subjects, excretion of parent aminolevulinic acid (ALA) in urine in the 12 hours following administration of the recommended dose of ALA solution was 34 + 8% (mean + std dev) with a range of 27% to 57%. In healthy volunteers, the administration of aminolevulinic acid resulted in a volume of distribution of 9.3 ± 2.8 L intravenously and 14.5 ± 2.5 orally.[11961050] Metabolism / Metabolites Exogenous aminolevulinic acid (ALA) is metabolized to PpIX, but the fraction of administered ALA that is metabolized to PpIX is unknown. The average plasma AUC of PpIX is less than 6% of that of ALA. Following topical administration, synthesis into protoporphyrin IX takes place in situ in the skin. Half Life: Mean half-life is 0.70 ± 0.18 h after the oral dose and 0.83 ± 0.05 h after the intravenous dose. Biological Half-Life The mean ± SD elimination half-life (t1/2) of aminolevulinic acid was 5.7 ± 3.9 hours for the topical solution formulation and the mean half-life was 0.9 ± 1.2 hours for the oral solution formulation. In another pharmacokinetic studies with 6 healthy volunteers using a 128 mg dose, the mean half-life was 0.70 ± 0.18 h after the oral dose and 0.83 ± 0.05 h after the intravenous dose. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
According to the presumed mechanism of action, photosensitization following application of aminolevulinic acid (ALA) topical solution occurs through the metabolic conversion of ALA to protoporphyrin IX (PpIX), which accumulates in the skin to which aminolevulinic acid has been applied. When exposed to light of appropriate wavelength and energy, the accumulated PpIX produces a photodynamic reaction, a cytotoxic process dependent upon the simultaneous presence of light and oxygen. The absorption of light results in an excited state of the porphyrin molecule, and subsequent spin transfer from PpIX to molecular oxygen generates singlet oxygen, which can further react to form superoxide and hydroxyl radicals. Photosensitization of actinic (solar) keratosis lesions using aminolevulinic acid, plus illumination with the BLU-UTM Blue Light Photodynamic Therapy Illuminator (BLU-U), is the basis for aminolevulinic acid photodynamic therapy (PDT). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the use of oral aminolevulinic acid during breastfeeding. To minimize exposure of the infant, breastfeeding can be withheld for 24 hours after an oral dose. Breastfeeding is not expected to result in exposure of the child to topical aminolevulinic acid due to negligible systemic absorption. Aminolevulinic acid-induced photodynamic therapy has been used successfully to treat various skin lesions of the nipple. This treatment appeared to preserve nipple anatomy for breastfeeding. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding In in vitro experiments using aminolevulinic acid (ALA) concentrations up to approximately 25% of the maximal concentration that occurs in plasma following the recommended dose of ALA solution, the mean protein binding of ALA was 12%. |
| 参考文献 | |
| 其他信息 |
Pharmacodynamics
The metabolism of aminolevulinic acid (ALA) is the first step in the biochemical pathway resulting in heme synthesis. Aminolevulinic acid is not a photosensitizer, but rather a metabolic precursor of protoporphyrin IX (PpIX), which is a photosensitizer. The synthesis of ALA is normally tightly controlled by feedback inhibition of the enzyme, ALA synthetase, presumably by intracellular heme levels. ALA, when provided to the cell, bypasses this control point and results in the accumulation of PpIX, which is converted into heme by ferrochelatase through the addition of iron to the PpIX nucleus. |
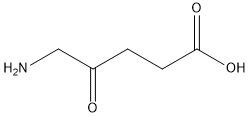
| 分子式 |
C5H9NO3
|
|---|---|
| 分子量 |
131.12986
|
| 精确质量 |
131.058
|
| 元素分析 |
C, 45.80; H, 6.92; N, 10.68; O, 36.60
|
| CAS号 |
106-60-5
|
| 相关CAS号 |
5-Aminolevulinic acid hydrochloride;5451-09-2;5-Aminolevulinic acid-13C;123253-93-0
|
| PubChem CID |
137
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
298.4±20.0 °C at 760 mmHg
|
| 熔点 |
156-158 °C
156 - 158 °C |
| 闪点 |
134.3±21.8 °C
|
| 蒸汽压 |
0.0±1.3 mmHg at 25°C
|
| 折射率 |
1.482
|
| LogP |
-0.93
|
| tPSA |
80.39
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
9
|
| 分子复杂度/Complexity |
121
|
| 定义原子立体中心数目 |
0
|
| SMILES |
NCC(=O)CCC(O)=O
|
| InChi Key |
ZGXJTSGNIOSYLO-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C5H9NO3/c6-3-4(7)1-2-5(8)9/h1-3,6H2,(H,8,9)
|
| 化学名 |
5-amino-4-oxopentanoic acid
|
| 别名 |
5-Aminolevulinic acid; Aminolevulinic acid; 106-60-5; 5-Amino-4-oxopentanoic acid; 5-Aminolevulinate; Pentanoic acid, 5-amino-4-oxo-; delta-aminolevulinic acid; Aladerm; 5451-09-2 (HCl); 106-60-5 (free); 868074-65-1 (phosphate)
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~762.60 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (19.07 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (19.07 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (19.07 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 7.6260 mL | 38.1301 mL | 76.2602 mL | |
| 5 mM | 1.5252 mL | 7.6260 mL | 15.2520 mL | |
| 10 mM | 0.7626 mL | 3.8130 mL | 7.6260 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。