| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g | |||
| 10g | |||
| 50g | |||
| Other Sizes |
| 靶点 |
endogenous purines
Hypoxanthine-guanine phosphoribosyltransferase (HGPRT) [1] Purine nucleotide synthetase [1] Orphan nuclear receptor NR4A3 (functional target for glucose transport regulation) [2] |
|---|---|
| 体外研究 (In Vitro) |
巯嘌呤广泛用于治疗恶性肿瘤、风湿性疾病、皮肤病、炎症性肠病和实体器官移植排斥反应。巯嘌呤通过抑制磷酸核糖焦磷酸酰胺转移酶(PRPP 酰胺转移酶)来抑制嘌呤核苷酸的合成和代谢。 PRPP酰胺转移酶是嘌呤合成的限速酶。它改变 RNA 和 DNA 的合成和功能。巯嘌呤干扰核苷酸互变和糖蛋白合成。
针对人急性淋巴细胞白血病(ALL)细胞和结直肠癌细胞,6-巯基嘌呤一水合物(6-MP)表现出浓度依赖性抗增殖活性,IC50值为10-50 μM。它抑制嘌呤核苷酸从头合成,并掺入DNA/RNA中,导致复制阻滞[1] - 在L6大鼠骨骼肌细胞中,6-巯基嘌呤一水合物(6-MP)(10-100 μM)以时间依赖方式增强葡萄糖转运活性2.0-2.5倍。该效应部分通过上调孤儿核受体NR4A3表达介导,促进GLUT4向细胞膜转运[2] - 在大鼠胎脑神经祖细胞(NPCs)中,6-巯基嘌呤一水合物(6-MP)(5-20 μM)诱导G1期细胞周期阻滞和凋亡。20 μM浓度下,NPCs活力降低60%,caspase-3激活增加,cyclin D1表达降低[3] |
| 体内研究 (In Vivo) |
在6-巯基嘌呤水合物(6-MP)治疗组的胎儿端脑中,S期细胞群在治疗后36和48小时增加,并在治疗后72小时恢复到对照水平。 G2/M期细胞群在24小时开始增加,36小时达到峰值,48小时减少,最后在72小时恢复到对照水平。另一方面,亚G1期细胞群(凋亡细胞)在36小时开始增加,在48小时达到峰值,然后在72小时减少。
在急性淋巴细胞白血病(ALL)患儿中,口服6-巯基嘌呤一水合物(6-MP) 1.5-2.5 mg/kg/天作为维持治疗,显著抑制白血病细胞增殖,5年无事件生存率达70-80%。药效与硫嘌呤S-甲基转移酶(TPMT)基因型相关[1] - 妊娠大鼠从妊娠第10天至第18天,通过灌胃口服6-巯基嘌呤一水合物(6-MP) 5 mg/kg/天,胎鼠大脑神经祖细胞增殖减少40%,凋亡增加2.3倍,导致大脑皮层发育受损[3] - 在自身免疫性关节炎小鼠模型中,口服6-巯基嘌呤一水合物(6-MP) 3 mg/kg/天,抑制T/B淋巴细胞活化,关节炎症和组织损伤减轻55%[1] |
| 酶活实验 |
L6 肌管在 DMSO 对照或 6-巯基嘌呤水合物 (6-MP) 中孵育 24 小时,最后 3 小时在无血清 DMEM 中进行处理。然后在存在或不存在 100 nM 胰岛素的情况下在 37°C 下再孵育 60 分钟。随后,收集 50 μg 蛋白质裂解物,进行 SDS-PAGE,然后使用一抗在 4°C 下进行一整夜的免疫印迹。使用 Image J 软件,对扫描胶片进行光密度分析,最终量化蛋白质 [2]。
HGPRT活性检测:将纯化的人HGPRT与次黄嘌呤和磷酸核糖焦磷酸(PRPP)在反应缓冲液中于37°C孵育。加入系列浓度(1-50 μM)的6-巯基嘌呤一水合物(6-MP),混合物孵育60分钟。加入三氯乙酸终止反应,通过高效液相色谱(HPLC)定量肌苷一磷酸(IMP)生成量,证实6-MP对HGPRT的竞争性抑制作用[1] - 嘌呤核苷酸合成酶抑制检测:将重组人人磷酸核糖胺-甘氨酸连接酶(GAR合成酶)与甘氨酸和磷酸核糖胺在反应缓冲液中孵育。加入5-100 μM的6-巯基嘌呤一水合物(6-MP),混合物在37°C孵育90分钟。基于茚三酮反应的比色法检测GAR(产物)生成量,定量酶抑制效果[1] |
| 细胞实验 |
细胞活力测定用于量化细胞活力。将 10,000 个 L6 骨骼肌细胞接种到 96 孔板的每孔中,7 天后,细胞分化为肌管。测定前,用不同剂量的 6-巯基嘌呤水合物 (6-MP) 处理细胞 24 小时。室温平衡 30 分钟后,向每孔中添加 50 μL Cell Titer-Glo 试剂,并将板在定轨摇床上混合 12 分钟以分析细胞的活力。光度计用于测量光度[2]。
白血病细胞抗增殖检测:将人ALL细胞以5×10³个细胞/孔接种到96孔板中,用1-100 μM的6-巯基嘌呤一水合物(6-MP)处理72小时。采用四唑盐比色法检测细胞活力,计算IC50值。通过放射性示踪法证实6-MP代谢产物掺入DNA/RNA[1] - 骨骼肌细胞葡萄糖转运检测:将L6骨骼肌细胞分化为肌管,用10-100 μM的6-巯基嘌呤一水合物(6-MP)处理24-48小时。采用[3H]-2-脱氧葡萄糖摄取实验检测葡萄糖转运活性。蛋白质印迹法检测NR4A3和GLUT4蛋白表达[2] - 神经祖细胞凋亡及周期检测:分离大鼠胎脑NPCs,以1×10⁴个细胞/孔接种到24孔板中。用5-20 μM的6-巯基嘌呤一水合物(6-MP)处理48小时。通过膜联蛋白V-FITC/PI染色和流式细胞术检测凋亡。碘化丙啶染色分析细胞周期分布,蛋白质印迹法定量cyclin D1/caspase-3水平[3] |
| 动物实验 |
In this study, pregnant rats that are about thirteen weeks old are employed. The animals are kept in separate wire-mesh cages in an air-conditioned room with constant temperature and humidity levels (23±3°C and 50±20%, respectively), 10 cycles of ventilation (lights on for 12 hours and dark for 12 hours), and free access to pelleted food and water. In the experiment, three dams are each sacrificed by exsanguination from the abdominal aorta under ether anesthesia at 12, 24, 36, 48, and 72 hours after fifteen pregnant rats receive an intraperitoneal injection of 50 mg/kg 6-Mercaptopurine hydrate (6-MP) on E13. Each dam's fetuses are removed via Caesarean section. Three dams are sacrificed at each of the same time points, and fifteen pregnant rats are injected intraperitoneally (i.p.) with a 2.0% methylcellulose solution in distilled water as controls at E13[3].
Fetal neural toxicity rat model: Pregnant Sprague-Dawley rats were randomly divided into control and treatment groups (n=8 per group). 6-Mercaptopurine (6-MP) Monohydrate was dissolved in sterile water and administered orally via gavage at 5 mg/kg/day from gestational day 10 to 18. On gestational day 19, rats were euthanized, and fetal brains were harvested for neural progenitor cell isolation, proliferation assay (BrdU incorporation), and apoptosis detection (TUNEL staining) [3] - Autoimmune arthritis murine model: C57BL/6 mice with collagen-induced arthritis were administered 6-Mercaptopurine (6-MP) Monohydrate (3 mg/kg/day) via oral gavage for 21 days. Joint inflammation was scored weekly, and spleen T/B lymphocyte activation was analyzed by flow cytometry (CD4+CD69+, CD19+CD69+) [1] |
| 药代性质 (ADME/PK) |
Absorption: 6-Mercaptopurine (6-MP) Monohydrate has variable oral bioavailability (50-70%) in humans, with peak plasma concentrations reached 1-2 hours after administration [1]
- Distribution: Distributes widely into tissues, with highest concentrations in bone marrow, liver, and spleen. Plasma protein binding rate is approximately 10-15% [1] - Metabolism: Primarily metabolized by thiopurine S-methyltransferase (TPMT) and xanthine oxidase (XO) in the liver. TPMT genotype polymorphism (homozygous variant, heterozygous, wild-type) significantly affects metabolic rate and drug accumulation [1] - Excretion: Approximately 40% of the administered dose is excreted in urine within 24 hours, mostly as metabolites [1] - Half-life: Plasma elimination half-life is 1-2 hours in humans, prolonged in TPMT-deficient individuals (up to 10 hours) [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation In the treatment of conditions such as ulcerative colitis and Crohn's disease, most professional guidelines and other experts consider breastfeeding to be acceptable during mercaptopurine therapy.[1-9] Azathioprine is rapidly converted to mercaptopurine, so data from mothers taking azathioprine apply to mercaptopurine. No active metabolites of mercaptopurine were found in the blood of breastfed infants whose mothers were taking azathioprine and only poorly documented cases of mild, asymptomatic neutropenia and increased rates of infection have been reported occasionally. It might be desirable to monitor exclusively breastfed infants with a complete blood count with differential, and liver function tests if azathioprine is used during lactation, although some authors feel that such monitoring is unnecessary.[10]. See the Azathioprine record for details. Mothers with decreased activity of the enzyme that detoxifies mercaptopurine metabolites may transmit higher levels of drug to their infants in breastmilk. It might be desirable to monitor exclusively breastfed infants with a complete blood count with differential, and liver function tests if mercaptopurine is used during lactation, although some authors feel that monitoring is unnecessary.[11] Avoiding breastfeeding for 4 hours after a dose should markedly decrease the dose received by the infant in breastmilk.[12] Most sources consider breastfeeding to be contraindicated during maternal antineoplastic drug therapy, although antimetabolites such as mercaptopurine appear to pose the least risk to breastfed infants.[13] After high-dose chemotherapy, it might be possible to breastfeed safely during intermittent therapy with an appropriate period of breastfeeding abstinence. Although no data are available to determine an appropriate period to withhold breastfeeding, the drug's terminal half-life suggests that withholding breastfeeding for 1 to 2 days may be sufficient. Chemotherapy may adversely affect the normal microbiome and chemical makeup of breastmilk.[14] ◉ Effects in Breastfed Infants In The Netherlands, 30 infants of mothers taking either azathioprine (n = 28) or mercaptopurine (n = 2) for inflammatory bowel disease during pregnancy and postpartum were followed at 1 to 6 years of age using a 43-item quality of life questionnaire. Of this cohort, 9 infants were breastfed for a mean of 7 months (range 3 to 13 months) No statistically significant differences were found between breastfed and formula-fed infants in any of the 12 domains of the survey.[19] In a multi-center study of women with inflammatory bowel disease in pregnancy (the PIANO registry), 102 women received a thiopurine (azathioprine or mercaptopurine) and another 67 received a thiopurine plus a biological agent (adalimumab, certolizumab, golimumab, infliximab, natalizumab, or ustekinumab) while breastfeeding their infants. Among those who received a thiopurine or combination therapy while breastfeeding, infant growth, development or infection rate was no different from 208 breastfed infants whose mothers received no treatment.[20] A national survey of gastroenterologists in Australia identified 21 infants who were breastfed by mothers taking a combination of allopurinol and a thiopurine (e.g. azathioprine, mercaptopurine) to treat inflammatory bowel disease. All had taken the combination during pregnancy also. Two postpartum infant deaths occurred, both at 3 months of age. One was a twin (premature birth-related) and the other from SIDS. The authors did not believe the deaths were medication related.[21] No information was provided on the extent of breastfeeding, drug dosages or the outcomes of the other infants. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Myelosuppression: The most common dose-limiting toxicity, characterized by leukopenia (incidence 30-40%) and thrombocytopenia (15-20%) in humans at therapeutic doses (1.5-2.5 mg/kg/day) [1] - Developmental neurotoxicity: In fetal rats, maternal exposure to 5 mg/kg/day caused reduced neural progenitor cell proliferation and increased apoptosis, leading to cerebral cortex hypoplasia [3] - Hepatic toxicity: Mild elevation of serum transaminases (1.5-2.0-fold) occurs in 10-15% of patients, reversible with dose reduction [1] - Gastrointestinal toxicity: Nausea, vomiting, and diarrhea (incidence 10-15%) in humans, more common with high doses [1] - Drug-drug interactions: Concurrent use with allopurinol (XO inhibitor) increases 6-MP plasma concentrations by 2-3-fold, requiring dose reduction; co-administration with methotrexate enhances myelosuppression [1] |
| 参考文献 |
|
| 其他信息 |
6-mercaptopurine monohydrate is an odorless light yellow to yellow crystalline powder. Becomes anhydrous at 284 °F. (NTP, 1992)
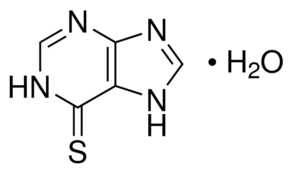
Mercaptopurine hydrate is a hydrate. It contains a mercaptopurine. Mercaptopurine is a thiopurine-derivative antimetabolite with antineoplastic and immunosuppressive activities. Produced through the metabolism of mercaptopurine by hypoxanthine-guanine phosphoribosyltransferase (HGPRT), mercaptopurine metabolites 6-thioguanosine-5'-phosphate (6-thioGMP) and 6-thioinosine monophosphate (T-IMP) inhibit nucleotide interconversions and de novo purine synthesis, thereby blocking the formation of purine nucleotides and inhibiting DNA synthesis. This agent is also incorporated into DNA in the form of deoxythioguanosine, which results in the disruption of DNA replication. In addition, mercaptopurine is converted to 6-methylmercaptopurine ribonucleoside (MMPR) by 6-thiopurine methyltransferase; MMPRs are also potent inhibitors of de novo purine synthesis. (NCI04) An antimetabolite antineoplastic agent with immunosuppressant properties. It interferes with nucleic acid synthesis by inhibiting purine metabolism and is used, usually in combination with other drugs, in the treatment of or in remission maintenance programs for leukemia. See also: Mercaptopurine (annotation moved to). Drug Indication Xaluprine is indicated for the treatment of acute lymphoblastic leukaemia (ALL) in adults, adolescents and children. 6-Mercaptopurine (6-MP) Monohydrate is a synthetic thiopurine antimetabolite, first approved for clinical use in the 1950s [1] - Mechanism of action: It is converted to active metabolites (6-thioguanosine monophosphate, 6-TGMP; 6-thioinosine monophosphate, 6-TIMP) via HGPRT. These metabolites inhibit de novo purine synthesis, incorporate into DNA/RNA to block replication/transcription, and suppress immune cell activation [1] - Clinical indications: Approved for the treatment of acute lymphoblastic leukemia (ALL), chronic myeloid leukemia (CML), and autoimmune diseases (rheumatoid arthritis, inflammatory bowel disease) [1] - Pharmacogenetics: TPMT gene polymorphism is a key predictor of toxicity—TPMT-deficient patients are at high risk of severe myelosuppression, requiring dose reduction or alternative therapy [1] - Additional biological activity: Regulates glucose transport in skeletal muscle via NR4A3, suggesting potential applications in metabolic disorders (e.g., type 2 diabetes) [2] |
| 分子式 |
C5H6N4OS
|
|
|---|---|---|
| 分子量 |
170.19
|
|
| 精确质量 |
170.026
|
|
| 元素分析 |
C, 35.29; H, 3.55; N, 32.92; O, 9.40; S, 18.84
|
|
| CAS号 |
6112-76-1
|
|
| 相关CAS号 |
50-44-2
|
|
| PubChem CID |
2724350
|
|
| 外观&性状 |
Light yellow to yellow solid powder
|
|
| 沸点 |
490.6ºC at 760 mmHg
|
|
| 熔点 |
>300 °C(lit.)
|
|
| 闪点 |
250.5ºC
|
|
| LogP |
0.951
|
|
| tPSA |
98.68
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
3
|
|
| 可旋转键数目(RBC) |
0
|
|
| 重原子数目 |
11
|
|
| 分子复杂度/Complexity |
190
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
S=C1C2=C(N=C([H])N2[H])N([H])C([H])=N1.O([H])[H]
|
|
| InChi Key |
WFFQYWAAEWLHJC-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C5H4N4S.H2O/c10-5-3-4(7-1-6-3)8-2-9-5;/h1-2H,(H2,6,7,8,9,10);1H2
|
|
| 化学名 |
3,7-dihydropurine-6-thione;hydrate
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (14.69 mM) (饱和度未知) in 10% DMSO + 40% PEG300 +5% Tween-80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80+,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 5.8758 mL | 29.3789 mL | 58.7579 mL | |
| 5 mM | 1.1752 mL | 5.8758 mL | 11.7516 mL | |
| 10 mM | 0.5876 mL | 2.9379 mL | 5.8758 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05506332 | Recruiting | Drug: 6-mercaptopurine Drug: Venetoclax |
Acute Myeloid Leukemia, in Relapse Acute Myeloid Leukemia Refractory |
University Hospital, Antwerp | July 15, 2022 | Phase 1 |
| NCT05276284 | Recruiting | Combination Product: Atezolizumab, 6-mercaptopurine, 6-thioguanine |
Solid Tumor, Adult Metastatic Cancer |
Kristoffer Rohrberg | September 1, 2022 | Phase 1 Phase 2 |
| NCT01432145 | Completed | Drug: 6-Mercaptopurine Drug: Methotrexate |
Breast Cancer Ovarian Cancer |
University of Oxford | May 2011 | Phase 2 |
| NCT01324336 | Completed | Drug: 6-Mercaptopurine | Acute Lymphoblastic Leukemia | Children's Mercy Hospital Kansas City |
July 2011 | N/A |
| NCT00548431 | Completed | Drug: 6-mercaptopurine | Leukemia, Lymphocytic, Acute | Rigshospitalet, Denmark | December 2007 | Phase 2 |
|
|
|
|