| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| Other Sizes |
| 靶点 |
Antiviral; SARS-CoV-2; Prodrug form of GS-441524
|
|---|---|
| 体外研究 (In Vitro) |
GS-621763在细胞系和人类原代细胞培养物中对严重急性呼吸系统综合征冠状病毒2型具有抗病毒活性。[1]
GS-441524是单磷酰胺前药RDV(GS-5734,图1B)和三酯前药GS-621763(图1C)的亲代腺苷核苷类似物(图1A)。这三种分子在细胞中代谢为相同的活性核苷酸三磷酸,但通过不同的激活途径。GS-621763在口服吸收过程中迅速代谢为GS-441524,然后在细胞内被细胞激酶转化为类似的单磷酸盐代谢物,然后进一步代谢为活性核苷三磷酸盐。相比之下,完整的磷酰胺前药RDV在细胞内直接分解为相同的单磷酸盐代谢物,有效地绕过了GS-441524的限速第一磷酸化步骤。为了确定GS-621763是否可以在细胞检测中抑制严重急性呼吸系统综合征冠状病毒2型的复制,我们首先在稳定表达人血管紧张素转换酶2(ACE2)的A549-hACE2细胞中评估了其对表达纳米萤光素酶的严重急性呼吸系综合征冠状病毒-2报告病毒(严重急性呼吸综合征冠状病毒-2nLUC)的抗病毒活性。使用GS-621763,我们观察到对严重急性呼吸系统综合征冠状病毒2型nLUC复制的剂量依赖性抗病毒作用,平均半最大有效浓度(EC50)为2.8μM(图1D、图1H和补充图1A)。在同一试验中,我们测量了对照化合物RDV的EC50值为0.28μM,与之前在这些细胞中报告的值相似,反映了磷酰胺前药通过绕过较慢的初始磷酸化步骤快速有效地产生活性三磷酸盐的能力增强(图1D、图1H、补充图1A)。正如在其他细胞系统中观察到的那样,在我们的检测中,亲本核苷GS-441524的效力(EC50=3.3μM)低于RDV,效力与GS-621763相似。这表明GS-621763的三异丁酰酯在测定中被有效裂解,释放出GS-441524(图1D、图1H、补充图1A)。重要的是,我们在浓度高达10μM的A549-hACE2细胞中没有观察到任何抑制剂的任何可测量的细胞毒性(图1F,补充图1B)。人原代气道上皮(HAE)细胞培养物模拟了人类传导气道的细胞复杂性和结构,通常用于确定药物是否在体内新出现的冠状病毒靶向的细胞中运输和代谢。在感染野生型严重急性呼吸系统综合征冠状病毒2型并用GS-621763处理的HAE细胞中,与DMSO载体处理的培养物相比,我们观察到感染性病毒产生呈剂量依赖性和显著减少(图1F)。在类似感染的对照化合物(即RDV或GS-441524)处理的培养物中,也观察到病毒滴度的显著和剂量依赖性降低(图1F)。GS-621763、RDV和GS-441524分别以0.125、0.0371和2.454μM的EC50值抑制了正常人支气管上皮(NHBE)培养物中表达报告基因SARS-CoV-2的萤火虫荧光素酶(SARS-CoV-2Fluc)的复制(图1G、图1H)。总之,这些数据表明,GS-621763是运输、代谢和强效抗病毒的人类原代细胞系统,该系统模拟了人类严重急性呼吸系统综合征冠状病毒2型的靶向组织。 严重急性呼吸系统综合征冠状病毒2感染的Vero E6细胞中的半数最大有效浓度(EC50)高度一致,GS-621763的范围为0.11至0.73μM(图1b,补充表1),GS-441524的范围为0.12至0.68μM(表1c,补充表一)。在稳定表达人ACE2的A549细胞(A549-hACE2)的剂量反应试验中检测表达萤光素酶的WA1/2020报告病毒时,获得了类似的效力范围(补充表1)20。不同细胞系和来源于不同供体的原代人类细胞中GS-621763、瑞德西韦和GS-441524的毒性测试显示,GS-621763在VeroE6细胞中的选择性指数(SI=CC50/EC50)>137,在A549-ACE2细胞中的选择指数(SI=3C50/EC50)分别为40至>100μM(图1d,补充表1)、36至>100微米(补充图1,补充表一)和>100微米。GS-441524和GS-621763的疗效在气液界面生长并顶部感染VOCγ的分化良好的原代人气道上皮培养物上进行了平行评估(图1f-g)。基底外侧添加的GS-441524或GS-621763在这种与疾病相关的人体组织模型中显示出类似的效力,分别返回2.83和3.01µM的EC50值。跨上皮电阻的平行测量表明,在基底外侧药物浓度≥3µM时,上皮完整性得到了充分保护[2]。 |
| 体内研究 (In Vivo) |
GS-621763在新冠肺炎疾病小鼠模型中的剂量依赖性治疗效果。[1]
我们之前进行了多项研究,描述了皮下注射RDV对小鼠(Ces1c-/-C57BL/6J)的治疗效果,这些小鼠因分泌的血浆羧酸酯酶1c(Ces1c)而基因缺失,该羧酸酯酶1c在人类中不存在,但大大降低了野生型小鼠的药物半衰期。[1] 然而,前药GS-621763被设计为在体内全身前快速切割,将GS-441524释放到循环中,血浆中没有或只有极少的完整酯。因此,GS-621763可以在野生型小鼠中进行研究,在那里它也应该迅速转化为亲本GS-441524。首次在未感染的BALB/c小鼠中测定了单次口服5或20mg/kg GS-621763后的血浆药代动力学(图2A)。选择剂量以提供GS-441524的高血浆暴露量,这将支持肺部活性三磷酸盐的形成,并确认在疗效研究中预测暴露量所需的药代动力学剂量比例。先前的研究表明,以摩尔计,母体核苷产生肺三磷酸的效率至少比RDV低10倍,因此需要更高的母体GS-441524血浆暴露量来解释代谢效率的降低。在检测限值内,未在小鼠中观察到完整酯前药的暴露。GS-441524被迅速吸收,然后从体循环中清除,表现出约1小时的短血浆半衰期。在两种剂量下,观察到最大血浆浓度(Cmax)和暴露量(AUC0-24h)的剂量成比例增加。[1] 为了更好地了解GS-621763的药代动力学和药效学关系,我们在感染了小鼠适应型严重急性呼吸系统综合征冠状病毒2型(严重急性呼吸综合征冠状病毒2-MA10)的BALB/c小鼠中进行了一系列剂量发现研究。在感染104个斑块形成单位(PFU)严重急性呼吸系统综合征冠状病毒2型MA10的年轻成年BALB/c小鼠中,病毒在呼吸道中复制到高滴度,感染后4天(dpi)小鼠体重减轻15-20%,通常在病毒复制达到2 dpi峰值后观察到急性肺损伤/肺功能丧失。我们首先确定了BALB/c小鼠在感染104 PFU严重急性呼吸系统综合征冠状病毒2型MA10后8小时(hpi)开始每天两次(即双待死,BID)口服载体对照或3 mg/kg、10 mg/kg或30 mg/kg GS-621763以获得最大疗效的最小剂量(图2B)。与载体或3 mg/kg GS-621763治疗的动物不同,接受10或30 mg/kg GS-6217 63的小鼠完全免于体重减轻,从而证明早期口服抗病毒治疗可以预防疾病进展(图2B)。与体重减轻表型一致,与赋形剂和3 mg/kg治疗组相比,10和30 mg/kg GS-621763治疗组的动物病毒肺滴度显著降低(图2C)。为了监测药物治疗对肺功能的影响,我们每天对每组的一部分小鼠进行全身体积描记术(WBP)(每组N=4只)。如WBP指标PenH所示,其升高与气道阻力或阻塞有关,我们观察到PenH的药物剂量依赖性降低,在30mg/kg GS-621763剂量组中效果最大,该剂量组完全免受其他治疗组和载体中观察到的肺功能丧失的影响(图2D)。用3和10 mg/kg GS-621763治疗的小鼠在感染后第2天和第3天肺功能受损,但所有GS-621763-治疗的动物的肺功能在4 dpi时恢复到基线水平(图2D)。与体重减轻、病毒滴度和肺功能数据一致,用10或30mg/kg治疗的小鼠肺充血明显减少,这是严重肺损伤的一个总体病理特征(图2E)。然后,我们使用两种互补的半定量工具对肺组织切片进行评分,以了解急性肺损伤(ALI)的组织学特征。首先,使用美国胸科学会(ATS)创建的ALI评分工具,我们对每个肺切片的三个病变区域进行了盲法评估,以确定ALI的几个特征,包括肺泡间隔增厚、间质和空气中的中性粒细胞、空气中的蛋白质碎片以及透明膜的存在。只有接受30mg/kg治疗的小鼠ALI评分显著降低(图2F)。其次,我们使用了一种补充工具来测量ALI的病理标志,即弥漫性肺泡损伤(DAD)。所有治疗组的小鼠都显示出DAD评分降低,但只有接受30mg/kg治疗的小鼠肺部DAD评分显著降低(图2G)。这些数据共同表明,口服核苷类似物GS-621763可以以剂量依赖的方式显著减少严重急性呼吸系统综合征冠状病毒2型病毒的复制和相关的肺部疾病。 GS-621763在小鼠中对新冠肺炎疾病的扩展治疗保护[1] 为了确定早期干预(感染后8小时)观察到的GS-621763的有效治疗效果是否会延伸到感染后的后期,我们设计了一项有六个臂的治疗效果研究,在感染严重急性呼吸系统综合征冠状病毒2型MA10的BALB/c小鼠中,我们改变了口服治疗开始的时间和剂量水平(图3)。如前所述,对照组动物从感染后12小时(hpi)开始每天接受两次赋形剂。该研究的接下来的三个组专门用于30 mg/kg GS-621763剂量水平,其中两个组每天接受两次给药,在12小时(“30 mg/kg BID 12小时”组)或24小时(“30mg/kg BID 24小时”组”)开始。第三个30 mg/kg组旨在确定如果在12 hpi时早期开始,剂量频率是否可以降低到每天一次(QD)(“30 mg/kg QD 12小时”组)。在最后两个组中,我们想评估从12小时或24小时开始每天一次增加60 mg/kg的剂量(“60 mg/kg每天一次12小时”和“60 mg/kg每日一次24小时”组)是否会改善30 mg/kg组的结果。在12或24小时开始30mg/kg BID治疗可显著防止体重减轻(图3A),延长了在很早的时间(8小时)开始时观察到的该剂量水平的稳健治疗表型(图2)。有趣的是,当我们将12小时开始的30mg/kg治疗频率降低到每天一次时(“30mg/kg QD 12小时组”),我们还观察到体重减轻的显著预防作用(图3A),因此每天一次并在感染过程中早期(12小时)开始给药的药物水平足以预防疾病进展。将剂量增加到60mg/kg QD,在12小时或24小时开始,与30mg/kg组相比,赋形剂治疗对体重减轻的保护作用相似(图3A)。 GS-621763的疗效与莫奈韦(MPV,EIDD-2801)相似[1] MPV是一种口服核苷类似物前药抗病毒药物,目前正在进行治疗新冠肺炎的3期临床试验,在小鼠中已证明其对几种新出现的CoV(包括SARS-CoV、MERS-CoV和SARS-CoV-2)的抗病毒功效。与GS-621763一样,MPV是一种前药,在体内代谢为亲代核苷(β-D-N4-羟基胞苷,NHC),在代谢过程中转化为抗病毒活性三磷酸盐。为了确定GS-621763是否会提供与MPV类似的保护,我们随后在上述严重急性呼吸系统综合征冠状病毒2型发病机制的小鼠模型中设计了比较疗效研究。使用MPV对BALB/c小鼠(30mg/kg或100mg/kg)进行了疗效前药代动力学研究,结果显示NHC血浆暴露量按剂量比例增加(补充图2)。药代动力学模型随后确定,每日120mg/kg剂量(给予60mg/kg BID)将导致与接受800mg BID的人体中观察到的暴露相似的暴露,该剂量正在人体临床试验中进行评估。比较疗效研究包括一个赋形剂组和另外5个组,每天接受两剂MPV或GS-621763,间隔12小时(BID)。该研究的三个组在12小时后开始给药:30mg/kg GS-621763、30mg/kg MPV(0.5×人体等效剂量)或60mg/kg MPV。24小时后,我们开始给药另外两组:60 mg/kg GS-621763或60 mg/kg MPV。虽然严重急性呼吸系统综合征冠状病毒2型MA10感染导致载体对照动物体重迅速下降,但所有在12或24小时开始接受GS-621763或MPV治疗的动物都受到了体重下降的保护(图4A)。同样,通过空斑试验在4 dpi下滴定肺组织中的感染性病毒后,载体处理的动物具有预期的高水平感染性病毒,在所有治疗组中都显著降低,与药物类型或起始时间无关(图4B)。当在12小时开始治疗时,在30mg/kg MPV组中观察到感染滴度中度但显著升高,低于同等剂量的GS-621763动物或接受更高剂量(60mg/kg)MPV的动物(图4B)。为了了解感染性病毒水平与肺组织中病毒RNA之间的关系,我们对用于斑块测定的平行组织中严重急性呼吸系统综合征冠状病毒2型N型RNA的总RNA进行了qRT-PCR。qRT-PCR数据反映了感染性病毒的趋势,其中所有接受抗病毒治疗的组的病毒RNA水平都显著降低(图4C)。此外,与同等剂量的GS-621763动物相比,接受30mg/kg MPV(0.5×人体等效剂量)的动物N RNA有可测量的增加。与体重减轻数据类似,在3和4 dpi时,通过WBP测量,载体治疗的动物肺功能明显丧失,这在所有接受抗病毒治疗的组中都得到了预防(图4D)。然后,我们使用上述两种互补的组织学工具对肺组织切片进行盲法评估,以了解ALI和DAD的病理表现。与上述数据一致,与赋形剂对照组相比,所有抗病毒治疗组的ALI评分均显著降低(图4E)。与ALI评分一致,与赋形剂治疗组相比,所有抗病毒治疗组的DAD组织学评分同样降低(图4F)。总之,这些数据表明,在早期或病毒复制高峰期(约24小时)开始使用GS-621763和MPV进行抗病毒治疗,可以显著减少病毒复制并改善疾病结果。 雪貂的预防效果[2] 为了测试抗病毒效果,我们用1×105个噬斑形成单位(pfu)的WA1/2020鼻内感染雪貂,然后每天两次(b.i.d.)口服20mg/kg体重的GS-621763,持续四天(图2b)。在感染时开始治疗,每隔12小时收集一次鼻腔灌洗液,并在感染后4天采集呼吸组织。感染后1.5天,经载体处理的动物鼻腔灌洗液中的严重急性呼吸系统综合征冠状病毒2型减载量达到稳定水平,约为1×104pfu/mL,而感染后12小时,仅在GS-621763治疗组的一只雪貂的灌洗液中可短暂检测到病毒(图2c)。雪貂模型的临床症状总体上很轻微6。然而,只有载体组的动物体温升高(图2d),体重增加减少(图2e)。感染后4天,从接受治疗的动物身上提取的鼻甲中检测不到病毒,而载体组动物的鼻甲负荷约为5×104pfu/g(图2f)。在灌洗液(图2g)和鼻甲(图2h)中发现的病毒RNA拷贝数反映了感染滴度结果,表明载体组和治疗组之间存在一致的统计学显著差异,分别为两个和三个数量级。与之前的研究6一致,下呼吸道中未检测到感染性病毒颗粒或病毒RNA(图2i,j)。 疗效和最低有效剂量[2] 为了在临床上更相关的治疗环境中确定最低有效剂量,我们在感染后12小时开始口服治疗,即首次在鼻腔灌洗液中检测到脱落病毒时,以10mg/kg和3mg/kg体重水平,每日两次给药(图3a)。按照相同的b.i.d.治疗方案,给予5mg/kg b.i.d.的EIDD-2801/molnupivir作为参考。EIDD-2801被列为参考化合物,因为在研究时,EIDD-2802是雪貂模型中唯一一种口服有效对抗严重急性呼吸系统综合征冠状病毒2型的核苷类似物。在治疗开始后12小时内,所有接受治疗的动物的脱落病毒载量均显著低于赋形剂组(图3b)。接受3mg/kg GS-621763的雪貂鼻腔灌洗液中的病毒载量比载体动物低约一个数量级,而接受10mg/kg GS-621763或EIDD-2801/molnupivir治疗的雪貂在感染后第3天将脱落减少到接近检测水平。与这种抑制作用相一致,用3 mg/kg GS-621763治疗可将鼻甲中的负担降低一个数量级(图3c),而10 mg/kgGS-621763和EIDD-2801/molnupivir治疗组的动物病毒负担接近检测限值。载体动物和任何治疗组之间的临床症状没有显著差异(图3d,e)。 抑制VOCγ的复制和传播[2] 为了探索GS-621763的抗严重急性呼吸系统综合征冠状病毒2型适应症谱,10 mg/kg GS-621763 b.i.d.在感染后12小时开始,在联合疗效和传播研究中最近出现了VOCγ22(图4a)。在最初的复制延迟后,感染后1.5天,在载体处理的动物中可以检测到脱落病毒,然后在感染后第2天迅速达到近104 pfu/mL的鼻腔灌洗稳定水平(图4b)。病毒RNA拷贝的定量模拟了感染滴度的特征,尽管感染后第一天的灌洗液中已经存在低病毒RNA载量(图4c)。感染后4天测定的鼻甲病毒滴度和RNA拷贝数同样很高,分别为104至105 pfu/g组织(图4d)和108至1010 RNA拷贝/g组织(图4e)。然而,在这些动物的肺部没有检测到传染性VOCγ病毒颗粒或病毒RNA(图4f,g),也没有出现体重变化或发烧等临床症状(补充图2a,b)。该演示模仿了我们之前对WA1/20206的经验,表明VOCγ不会比WA1/2020更积极地入侵雪貂宿主。口服GS-621763治疗VOCγ感染非常有效,将脱落的病毒负荷和组织滴度降低到无法检测的水平(图4b,d),并将鼻腔灌洗液和鼻甲中的病毒RNA拷贝数降低了三个数量级以上(图4c,e)。 |
| 酶活实验 |
抗病毒活性的体外检测[1]
感染前24小时,将A549-hACE2细胞以20000个细胞/孔/100μl的密度铺在黑壁透明底部96孔板上。将化合物GS-621763、GS-5734、GS-441524在100%DMSO(1:3)中稀释,得到10至0.002mM(最终10至0.002μM)的1000X剂量反应。所有条件均一式三份。在BSL3,去除培养基,在37°C下用100μl SARS-CoV-2 nLUC(MOI 0.008)感染细胞1小时,然后去除病毒,用感染培养基(DMEM、4%FBS、1X抗生素/抗真菌剂)洗涤孔(150μl),并加入含有药物剂量反应的感染培养基。将培养板在37°C下孵育48小时。每48小时进行一次NanoGlo测定。处理后48小时,姐妹板暴露于药物但未感染,通过CellTiter-Glo测定来评估细胞毒性。 |
| 细胞实验 |
细胞毒性试验[2]
在96孔板的每个孔中,接种7500个细胞。将细胞与最大浓度为100µM的三倍连续稀释的化合物一起孵育。每个板包括4孔阳性(100µM环己酰亚胺)和阴性(载体(0.2%二甲亚砜(DMSO))对照,用于归一化。将板在37°C和5%CO2的加湿室中孵育72小时。在每个孔中加入PrestoBlue™细胞活力试剂(10μl/孔),孵育1小时后在Synergy H1多模式微孔板读数器上记录荧光(激发560 nm,发射590 nm)。原始数据用公式归一化:细胞存活率=100×(信号样本-信号阳性对照)/(信号阴性对照-信号阳性控制)。使用Prism 9.1.0 for MacOS(GraphPad)中的抑制剂与归一化反应方程确定非线性回归后的50%细胞毒性浓度(CC50)和95%置信区间。对于A549-hACE2细胞的细胞毒性试验,在将5000个A549-hACE2细胞/孔接种到40µl培养基中之前,将化合物(200 nl)点样到384孔板上。将平板在37°C和5%CO2中孵育48小时。在第2天,加入40µl CellTiter Glo并混合5次。在Envision上读取板的发光情况,并使用非线性四参数回归模型计算CC50值。 病毒产量降低[2] 在感染前16小时的12孔板中,每孔接种2×105个VeroE6细胞。然后,在37°C下以0.1 pfu/细胞的感染复数(MOI)用指定的病毒感染融合的单层1小时,并频繁摇晃。取下接种物,用1毫升含有2%FBS和指定浓度化合物的DMEM代替。细胞在37°C和5%CO2下孵育48小时。收集上清液,等分并储存在-80°C下,然后通过空斑试验进行分析。 报告病毒检测[2] 将A549-hACE2细胞(在含有2%FBS的培养基中每孔12000个细胞)以50µl的体积镀入白色透明底96孔板中。第二天,使用帝肯D300e数字液体分配器将化合物以3倍的连续稀释液直接加入培养物中,DMSO体积标准化为最高化合物浓度(最终DMSO浓度<0.1%)。将稀释的化合物溶液与50μl表达纳米萤光素酶报告蛋白的严重急性呼吸系统综合征冠状病毒2-Nluc(MOI 0.025 pfu/细胞)混合。感染后48小时,向每个孔中加入75μl纳米萤光素酶底物溶液。使用Envision酶标仪测量萤光素酶信号。通过将化合物处理组的萤光素酶信号归一化为DMSO处理组的信号(设置为100%)来计算相对萤光素酶的信号。使用非线性四参数变量斜率回归模型计算EC50值。 气液界面(ALI)人气道上皮细胞(HAE)病毒滴度降低[2] 在第三代,每平方厘米约有150000个活的“F3”细胞接种在孔径为0.4µm的Transwell 6.5 mm聚酯膜插入物上。达到汇合点后(喂食后第4天),移除基础培养基,用PneumaClt-Ex Plus替换,同时移除顶端培养基以形成气液界面。ALI后约3周,纤毛跳动、粘液分泌和经上皮电阻(TEER)>300 Ohm*cm2明显,证实分化成功。在感染前5个月,每周用PBS对粘液进行顶端清洗,使细胞保持分化状态。感染前一小时,用150µl PBS测量TEER,用含有GS-621763或GS-441524或载体(0.1%二甲亚砜)的指定系列稀释液(从10µM减少三倍)的新鲜培养基替换基础培养基。顶端侧感染了在Calu-3细胞(谱系P.1.,分离株hCoV-19/日本/TY7-503/2021(BZ/2021;巴西P.1)上生长的约25000 PFU的严重急性呼吸系统综合征冠状病毒2型γ分离株,在37°C下在100µl DMEM中感染1小时,然后取出接种物并用PBS洗涤两次。在最终的TEER测量之前,细胞在37°C下孵育3天,并用10%中性缓冲福尔马林固定1小时。感染后48小时和72小时,用200µl PBS收集脱落顶端病毒30分钟,并通过空斑试验估算病毒滴度。为了确定EC50s,使用病毒滴度的平均顶部平台对对数病毒滴度进行归一化,以定义100%,并使用Prism 9.0.1 for MacOS的可变斜率非线性回归进行分析。TEER用EVOM或EVOM3系统测量。 |
| 动物实验 |
Small molecule drug synthesis and formulation [1]
Small molecules (GS-621763, RDV, Molnupiravir, and GS-441524) were solubilized in 100% DMSO for in vitro studies and in vehicle containing 2.5% DMSO; 10% Kolliphor HS-15; 10% Labrasol; 2.5% Propylene glycol; 75% Water (final formulation pH 2) (for GS-621763) and in vehicle containing 2.5% Kolliphor RH-40, 10% Polyethylene glycol 300, 87.5% Water (for MPV) for in vivo studies. In vivo plasma pharmacokinetic analysis of GS-621763 and molnupiravir (MPV) [1] Mice were orally administered either a single dose of GS-621763 (in vehicle containing 2.5% DMSO; 10% Kolliphor HS-15; 10% Labrasol; 2.5% Propylene glycol; 75% Water (final formulation pH 2) or two doses of molnupiravir (in vehicle containing 2.5% Kolliphor RH-40, 10% Polyethylene glycol 300, 87.5% Water) (BID, 12 hours apart). GS-621763 was given at either 5 or 20 mg/kg and MPV at either 30 or 100 mg/kg. Plasma was serially isolated from 4 mice at 0.25, 1, 2, 8 and 24 hrs post GS-621763 administration. Plasma was isolated from alternating groups of 4 mice per timepoint at 0.5, 2, 6, 12 (pre-second dose), 12.5, 18 and 24 hrs post MPV administration. 20 μl of plasma was added to a mixture containing 250 μl of methanol and 25 μL of internal standard solution and centrifuged. 250 μl of resulting supernatant was then transferred, filtered (Agilent Captiva 96, 0.2 μm) and dried under a stream of nitrogen at 40 °C. Following reconstitution in a mixture of 5% acetonitrile and 95% water, a 10 μl aliquot was injected onto an LC-MS/MS system. Plasma concentrations of either GS-621763 and GS-441524 or MPV and N-hydroxycytidine (NHC) were determined using 8 to 10-point calibration curves spanning at least 3 orders of magnitude with quality control samples to ensure accuracy and precision, prepared in normal mouse plasma. Analytes were separated by a 50 mm × 3.0 mm, 2.55 μm Synergi Polar-RP column (Phenomenex) using a multi-stage linear gradient from 5% to 95% acetonitrile in mobile phase A at a flow rate of 1 ml/min. Quantitation of GS-441524 metabolites in the lung following oral GS-621763 administration in Balb/c mice [1] Lungs from all mice administered GS-621763 were quickly isolated at 24 hrs post-dose and immediately snap frozen in liquid nitrogen. On dry ice, frozen lung samples were pulverized and weighed. Dry ice-cold extraction buffer containing 0.1% potassium hydroxide and 67 mM ethylenediamine tetraacetic acid (EDTA) in 70% methanol, containing 0.5μM chloro-adenosine triphosphate as internal standard was added and homogenized. After centrifugation at 20,000 × g for 20 minutes, supernatants were transferred and dried in a centrifuging evaporator. Dried samples were then reconstituted with 60 μL of mobile phase A, containing 3 mM ammonium formate (pH 5) with 10 mM dimethylhexylamine (DMH) in water, centrifuged at 20,000 × g for 20 minutes and final supernatants transferred to HPLC injection vials. An aliquot of 10 μl was subsequently injected onto an API 6500 LC/MS/MS system for analysis of GS-441524 and its phosphorylated metabolites, performed using a similar method as described previously. Pharmacokinetics [2] Female ferrets were either intravenously administered 10 mg/kg remdesivir as a 30-min infusion or orally administered 30 mg/kg GS-621763, after which plasma was isolated at 7–9 timepoints postadministration. Plasma samples underwent methanol protein precipitation followed by centrifugation. The resulting supernatants were isolated, evaporated to dryness under nitrogen and reconstituted with 5% acetonitrile for injection onto an LC-MS/MS system. Concentrations of remdesivir, GS-621763, and GS-441524 were determined using 9-point calibration curves spanning at least 3 orders of magnitude, with quality control samples to ensure accuracy and precision, prepared in normal ferret plasma. Analytes were separated by a 50 × 3.0 mm, 2.55 μm Synergi Polar-RP 30 A column using a mobile phase A consisting of 10 mM ammonium formate with 0.1% formic acid and a mobile phase B consisting of 0.1% formic acid in acetonitrile. A multi-stage linear gradient from 5% to 95% mobile phase B at a flow rate of 1 mL/min was employed for analyte separation. Pharmacokinetic parameters were calculated using Phoenix WinNonlin (version 8.2, Certara) and concentration-time profiles generated using Prism. Ferret lungs were collected at 24 h following initiation of drug administration. Whole tissues were quickly isolated and immediately placed into liquid nitrogen and stored at -80 °C until processing and LC-MS/MS analysis2. Reported values for lung total nucleosides are the sum of (GS-441524 and mono-, di-, and triphosphate (GS-443902) metabolites). Ferret efficacy studies [2] Female ferrets (6–10 months old, Mustela putorius furo) were purchased from Triple F Farms. Ferrets were rested for 7 days after arrival. Ferrets were then housed individually or in groups of 2 in ventilated negative-pressure cages in an ABSL-3 facility. Based on the previous experiments6, ferrets were randomly assigned to groups (n = 4) and used as an in vivo model to examine the efficacy of orally administered compounds against SARS-CoV-2 infection. No blinding of investigators was performed. Ferrets were anesthetized using dexmedetomidine/ketamine and infected intranasally with 1 × 105 pfu 2019-nCoV/USA-WA1/2020 in 1 mL (0.5 mL per nare). Body weight and rectal temperature were measured once daily. Nasal lavages were performed twice daily using 1 mL sterile PBS (containing Antibiotic-Antimycotic). Nasal lavage samples were stored at -80 °C until virus titration could be performed by plaque assay. Treatment (once daily (q.d.) or twice daily (b.i.d.)) was initiated at either 0 or 12 h after infection and continued until 4 days postinfection with either vehicle (2.5% dimethyl sulfoxide; 10% Kolliphor HS-15; 10% Labrasol; 2.5% propylene glycol; 75% water) or compound. Four days after infection, ferrets were euthanized, and tissues and organs were harvested and stored at -80 °C until processed. Contact transmission in ferrets [2] Eight ferrets were anesthetized and inoculated intranasally with 1 × 105 pfu of hCoV-19/Japan/TY7-503/2021. Twelve hours after infection, ferrets were split into two groups (n = 4; 2 ferrets per cage) and treated with vehicle or GS-621763 (10 mg kg−1) twice daily (b.i.d.) via oral gavage. At 54 h after infection, uninfected and untreated contact ferrets (two contacts for GS-621763; three contacts for vehicle) were co-housed with source ferrets. Co-housing was continued until 96 h after infection and source ferrets were euthanized. Contact ferrets were housed individually and monitored for an additional 4 days after separation from source ferrets and subsequently euthanized. Nasal lavages were performed on all source ferrets every 12 h and all contact ferrets every 24 h. For all ferrets, nasal turbinates and lung tissues were harvested to determine viral titers and the detection of viral RNA. |
| 药代性质 (ADME/PK) |
Plasma pharmacokinetics following a single oral administration of GS-621763 at either 5 or 20 mg/kg were first determined in uninfected BALB/c mice (Fig. 2A). Doses were selected to provide high plasma exposures of GS-441524 that would support active triphosphate formation in the lung and to confirm pharmacokinetic dose proportionality needed to project exposures in efficacy studies. Previous studies had shown that parent nucleoside was at least 10-fold less efficient at generating lung triphosphate than RDV, on a molar basis, thus requiring higher plasma exposures of parental GS-441524 to account for the reduced metabolic efficiency. No exposure of intact ester prodrug, within the limit of detection, was observed in mice. GS-441524 was both rapidly absorbed and then cleared from systemic circulation, exhibiting a short plasma half-life of approximately 1 hr. Dose proportional increases in both maximal plasma concentrations (Cmax) and exposures (AUC0–24h) at the two doses were observed (Fig. 2A). [1]
Pharmacokinetics following oral administration [2] Assessment of GS-621763 plasma PK parameters in the ferret revealed excellent oral bioavailability (Fig. 2a), extensive cleavage presystemically to generate high exposures of GS-441524 in the blood (Supplementary Table 2), efficient distribution to soft tissues of the respiratory system (lung), and confirmed anabolism to bioactive GS-443902 (Supplementary Table 3). Following a single 30 mg/kg oral dose of GS-621763 in ferrets, the daily systemic exposure (AUC0-24h) of GS-441524 was 81 µM.h, 4.5 fold higher than the exposure following IV remdesivir at 10 mg/kg and approximately 10-fold greater than that observed following an 200/100 mg IV remdesivir dose in human. Lower levels of bioactive GS-443902 were formed from oral 30 mg/kg GS-621763 dosing compared to 10 mg/kg IV remdesivir (Supplementary Table 3), illustrating the difference in intracellular activation efficiency of the phosphoramidate prodrug remdesivir compared to systemic parent nucleoside GS-441524. |
| 参考文献 | |
| 其他信息 |
The COVID-19 pandemic remains uncontrolled despite the rapid rollout of safe and effective SARS-CoV-2 vaccines, underscoring the need to develop highly effective antivirals. In the setting of waning immunity from infection and vaccination, breakthrough infections are becoming increasingly common and treatment options remain limited. Additionally, the emergence of SARS-CoV-2 variants of concern with their potential to escape therapeutic monoclonal antibodies emphasizes the need to develop second-generation oral antivirals targeting highly conserved viral proteins that can be rapidly deployed to outpatients. Here, we demonstrate the in vitro antiviral activity and in vivo therapeutic efficacy of GS-621763, an orally bioavailable prodrug of GS-441524, the parental nucleoside of remdesivir, which targets the highly conserved RNA-dependent RNA polymerase. GS-621763 exhibited significant antiviral activity in lung cell lines and two different human primary lung cell culture systems. The dose-proportional pharmacokinetic profile observed after oral administration of GS-621763 translated to dose-dependent antiviral activity in mice infected with SARS-CoV-2. Therapeutic GS-621763 significantly reduced viral load, lung pathology, and improved pulmonary function in COVID-19 mouse model. A direct comparison of GS-621763 with molnupiravir, an oral nucleoside analog antiviral currently in human clinical trial, proved both drugs to be similarly efficacious. These data demonstrate that therapy with oral prodrugs of remdesivir can significantly improve outcomes in SARS-CoV-2 infected mice. Thus, GS-621763 supports the exploration of GS-441524 oral prodrugs for the treatment of COVID-19 in humans.
In summary, we provide preclinical data demonstrating the in vitro antiviral activity and in vivo therapeutic efficacy of an orally bioavailable nucleoside analog prodrug, GS-621763. The data provided herein supports the future evaluation of orally bioavailable prodrugs of GS-441524 in humans with COVID-19. If safe and effective, this class of RdRp inhibitors could become part of the arsenal of existing oral antivirals that are desperately needed to address a global unmet need for the COVID-19 pandemic and CoV pandemics of the future. [1] Remdesivir is an antiviral approved for COVID-19 treatment, but its wider use is limited by intravenous delivery. An orally bioavailable remdesivir analog may boost therapeutic benefit by facilitating early administration to non-hospitalized patients. This study characterizes the anti-SARS-CoV-2 efficacy of GS-621763, an oral prodrug of remdesivir parent nucleoside GS-441524. Both GS-621763 and GS-441524 inhibit SARS-CoV-2, including variants of concern (VOC) in cell culture and human airway epithelium organoids. Oral GS-621763 is efficiently converted to plasma metabolite GS-441524, and in lungs to the triphosphate metabolite identical to that generated by remdesivir, demonstrating a consistent mechanism of activity. Twice-daily oral administration of 10 mg/kg GS-621763 reduces SARS-CoV-2 burden to near-undetectable levels in ferrets. When dosed therapeutically against VOC P.1 gamma γ, oral GS-621763 blocks virus replication and prevents transmission to untreated contact animals. These results demonstrate therapeutic efficacy of a much-needed orally bioavailable analog of remdesivir in a relevant animal model of SARS-CoV-2 infection. [2] |
| 分子式 |
C24H31N5O7
|
|---|---|
| 分子量 |
501.5322
|
| 精确质量 |
501.222
|
| 元素分析 |
C, 57.48; H, 6.23; N, 13.96; O, 22.33
|
| CAS号 |
2647442-13-3
|
| 相关CAS号 |
Mindeudesivir;2647442-33-7
|
| PubChem CID |
162625114
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
2.3
|
| tPSA |
168
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
11
|
| 可旋转键数目(RBC) |
11
|
| 重原子数目 |
36
|
| 分子复杂度/Complexity |
887
|
| 定义原子立体中心数目 |
4
|
| SMILES |
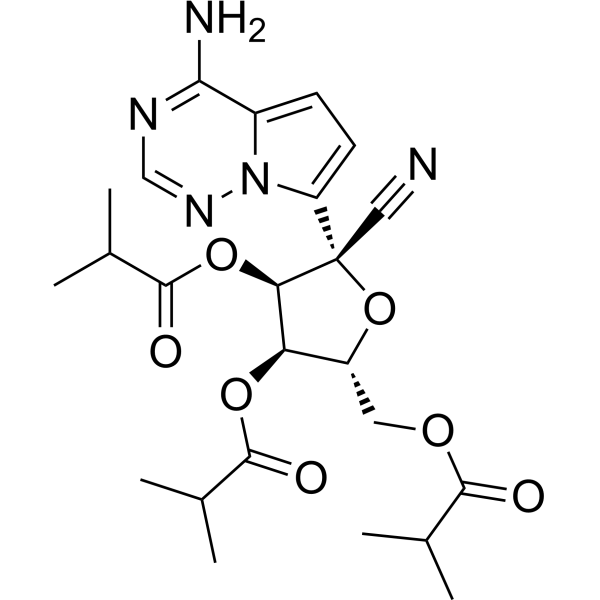
O1[C@]([H])(C([H])([H])OC(C([H])(C([H])([H])[H])C([H])([H])[H])=O)[C@]([H])([C@]([H])([C@]1(C#N)C1=C([H])C([H])=C2C(N([H])[H])=NC([H])=NN12)OC(C([H])(C([H])([H])[H])C([H])([H])[H])=O)OC(C([H])(C([H])([H])[H])C([H])([H])[H])=O
|
| InChi Key |
RVSSLHFYCSUAHY-JQGROFRJSA-N
|
| InChi Code |
InChI=1S/C24H31N5O7/c1-12(2)21(30)33-9-16-18(34-22(31)13(3)4)19(35-23(32)14(5)6)24(10-25,36-16)17-8-7-15-20(26)27-11-28-29(15)17/h7-8,11-14,16,18-19H,9H2,1-6H3,(H2,26,27,28)/t16-,18-,19-,24+/m1/s1
|
| 化学名 |
[(2R,3R,4R,5R)-5-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-5-cyano-3,4-bis(2-methylpropanoyloxy)oxolan-2-yl]methyl 2-methylpropanoate
|
| 别名 |
GS-621763; 2647442-13-3; GS-441524 prodrug; Remdesivir derivative; 83BU3492RP; GS621763; VV-116 free base; (2R,3R,4R,5R)-2-(4-Aminopyrrolo(2,1-f)(1,2,4)triazin-7-yl)-2-cyano-5-((isobutyryloxy)methyl)tetrahydrofuran-3,4-diyl bis(2-methylpropanoate);
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮和光照。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : 100 mg/mL (199.39 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (4.98 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 2.5 mg/mL (4.98 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (4.98 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9939 mL | 9.9695 mL | 19.9390 mL | |
| 5 mM | 0.3988 mL | 1.9939 mL | 3.9878 mL | |
| 10 mM | 0.1994 mL | 0.9969 mL | 1.9939 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。