| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| Other Sizes |
| 靶点 |
Antiviral; SARS-CoV-2; RSV; deuterated form of GS-621763
|
|---|---|
| 体外研究 (In Vitro) |
三异丁酸酯VV116还可以抑制RSV复制(EC50=1.20±0.32μM,CC50=95.92±9.27μM,SI=80,EC90=3.08±1.253μM),这表明VV116的酯部分易被细胞酶水解以释放母体核苷。这些化合物的抗RSV活性也在HEp-2和NHBE细胞中得到证实,HEp-2细胞和NHBE是RSV的其他许可细胞。[1]
药物化合物包括碳、氢和其他元素的稳定重同位素,主要作为影响药物开发过程中测量的示踪剂。药物的药代动力学和功能范围可能导致人们对突变的担忧[1]。延迟生成的化合物的潜在好处包括:(1)延迟生成的化合物可能能够扩展化合物的药代动力学特征,从而延长化合物的安全性、耐受性并改善耐受性; (2)延迟生成的化合物可以扩大肠道生物利用度。氘代化合物可能能够减少结肠和肠壁所需的首过代谢量,这将使更高比例的药物达到高生物利用度水平,这决定了其在低剂量下的疗效和更好的耐受性。 (3)增强新陈代谢功能。药物安全性、药物代谢 (4) 以及危险或反应性代谢物减少都是代谢物的潜在好处。氘代化学物质是无害的,并且有可能减轻或完全消除药物的负面影响。 (5)保存药用品质。根据早期的研究,氘代分子应保持与同类氢化合物相似的生物化学性质。 |
| 体内研究 (In Vivo) |
Mindeudesivir(VV116)(25、50和100 mg/kg;口服;每日两次,持续4天)显示出更强的活性,并将病毒滴度降至50 mg/kg的检测限以下,还可以减少呼吸道合胞病毒感染后的肺损伤[1]。VV116(25、50和100mg/kg;口服;单剂量)具有良好的PK特性和良好的安全性[1]。VV116(JT001)在Balb/c小鼠体内的药代动力学参数[1]。口服(25mg/kg)口服(50mg/kg)口服(100mg/kg)的Tmax(h)为0.42±0.14 0.42±0.12±0.14 Cmax(ng/mL)5360±560 11617±3443 24017±6521 AUC0-t(ng/mL·h)11461±1013 24594±1059 47799±6545 AUC0-∞(ng/mL-·h)11534±992 24739±1028 48014±6696 MRT0-∞(ng/mL·h)2.25±0.32 2.15±0.26 2.28±0.53 Tmax(小时)2.30±1.10 3.27±1.92 4.25±0.53动物模型:Balb/c小鼠[1]剂量:25、50和100mg/kg给药:口服;单剂量(药代动力学分析)结果:表现出良好的PK特性和良好的安全性。动物模型:Balb/c小鼠(6-8周;鼻内感染4×10^6 FFU的呼吸道合胞病毒)[1]剂量:25、50和100 mg/kg给药:口服;结果:在50mg/kg的剂量下,表现出更强的活性,病毒滴度降至检测限以下,也减少了呼吸道合胞病毒感染后的肺损伤。
考虑到Mindeudesivir(VV116)在体外抑制RSV的强效作用,我们在小鼠模型中进一步测试了VV16对RSV的影响。利巴韦林是临床上用于治疗呼吸道合胞病毒的标示外药物,被用作对照。为此,每只6-8周龄的Balb/c小鼠经鼻感染4×106 FFU的呼吸道合胞病毒(第0天),然后用VV116(25、50和100 mg/kg)或利巴韦林(50和100毫克/千克)双in die(b.i.d.)治疗(补充图S3)。我们之前的研究表明,在感染后第4天(p.i.),RSV感染的小鼠的病毒载量和病理学都达到了很高的水平,因此在这个时间点,小鼠被杀死,肺部被取出。用定量RT-PCR测量肺中的病毒RNA水平,用免疫斑点试验测量病毒颗粒载量(图1e)。值得注意的是,低剂量的VV116(25mg/kg)显示出与100mg/kg利巴韦林相当的抗病毒效果,分别将病毒RNA拷贝数和感染滴度降低了约1.5log10和约2.0log10(图1e)。中等剂量(50mg/kg)的VV116表现出更强的活性,并将病毒滴度降至检测限以下(图1e)。我们还通过组织化学分析评估了受攻击小鼠的肺部病理。呼吸道合胞病毒感染后,用赋形剂治疗的小鼠表现出严重的炎症,并出现肺泡炎性斑块。相比之下,在用VV116治疗的小鼠中只观察到轻微的肺浸润,表明VV116治疗可以减少呼吸道合胞病毒感染后的肺损伤[1]。 Balb/c小鼠的PK研究表明,在25至100mg/kg的剂量范围内,Mindeudesivir(VV116)具有线性PK曲线(图1c,补充表S6)。由于酯酶敏感前药的首过代谢,即使在100℃时,小鼠血浆中也未检测到VV116 口服给药后,母体核苷X1的血液浓度在0.5小时内迅速达到Cmax,在25mg/kg的剂量下,平均Cmax达到5360ng/ml(18.4µM,图1c,补充表S6、S7),远高于体外EC90值。X1的消除半衰期较短(2.3-4.25小时,补充表S6),支持每日两次给药方案。VV116的酯前药形式不仅旨在改善口服吸附,而且旨在规避核苷磷酰胺前药的肝脏靶向问题。临床前组织分布研究表明,X1在SD大鼠组织中广泛分布,5在Balb/c小鼠中也观察到X1的良好分布,X1在肺部的浓度约为肝脏的一半(图1d,补充表S8)。关于VV116的治疗窗口,大鼠14天重复给药口服毒性研究显示,无观测不良反应水平(NOAEL)为200mg/kg,此时X1的AUC0-t达到85151 ng h/ml(补充表S9),约为小鼠50mg/kg剂量的3.5倍。[1] |
| 酶活实验 |
抗病毒活性和细胞毒性测定[1]
将A549、HEp-2或NHBE细胞接种到48孔板中并孵育过夜。在达到80%的细胞融合后,在细胞与不同浓度的药物孵育1小时后,将细胞感染RSV A2(A549中的MOI为2,HEp-2中的MOI0.5,NHBE细胞中的MOI/1)2小时。然后,去除病毒-药物混合物,用含药物的培养基培养细胞。接种后48小时,从细胞中提取总RNA,然后使用带有gDNA橡皮擦的PrimeScript RT试剂盒进行逆转录。为了确定病毒拷贝数,使用TB Green®Premix Ex TaqTM II进行了绝对定量RT-PCR。RSV A2 F片段用引物5'-CGAGCACAGAGAGAACTACCA-3'定量;以及5'-CCTTCTAGGTGCAGGACCTTA-3'. 在96孔板中进行细胞存活率测定,每种浓度取三份。所有药物在维持培养基(含2%FBS的DMEM)中以500微摩尔开始,用9个梯度稀释2倍。孵育48小时后,去除上清液,并在培养基中加入10μL WST-8(2-(2-甲氧基-4-(苯基)-3-(4-(苯基)-5(2,4-磺基苯)-2h-四唑鎓单钠盐)的维持培养基。孵育2小时后,使用分光光度计(BioTek)在450nm波长下测量板,并计算细胞存活率。 |
| 细胞实验 |
细胞活力测定
细胞类型: A549(感染 RSV)[1] 测试浓度: 0-1000 μM 孵育时间:48小时 实验结果:抑制A549细胞中RSV复制,EC50为1.20±0.32 μM,CC50为95.92。 ± 9.27 μM,选择性指数 (SI) 80。 细胞和病毒[1] 本研究中使用的所有细胞均在37℃、5%CO2的加湿培养箱中培养。人喉表皮样癌(HEp-2)细胞、Vero E6细胞和A549细胞在添加了10%胎牛血清的Dulbocco的Medified Eagle培养基(DMEM;Gibco)中生长。正常人支气管上皮(NHBE)细胞在支气管上皮细胞生长培养基(BEGM)中维持,并在BulletKit中提供所有补充剂。 RSV A2株在HEp-2细胞中生长。感染后3或4天,从感染细胞中收集病毒。简而言之,将RSV感染的细胞重复冷冻和解冻3次,然后在4℃下以1000rpm离心10分钟。然后,收集上清液并在-80℃下储存直至使用。如前所述,通过免疫斑点试验测定Vero E6细胞中RSV A2的病毒滴度1。所有RSV A2感染实验均在生物安全二级(BSL-2)实验室进行。 |
| 动物实验 |
In vivo efficacy of Mindeudesivir (VV116) against RSV in mice [1]
Specific pathogen-free (SPF) female Balb/c mice at the age of 6–8 weeks were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. The mice were housed in an SPF environment under standard conditions. All mouse experiments were approved by the ethics committee of the Wuhan Institute of Virology, Chinese Academy of Science (permit number WIVA25202113). Thirty mice (5 animals per group, 6 groups) were anaesthetized with isoflurane and challenged with 4×106 FFU of RSV A2 intranasally (i.n.). The mice were given drugs by intragastric administration. Treatments were commenced in 1 h post infection and continued for 4 days. Mice in the control group were given the solvent (40% PEG 400+10% HS 15+50% ultrapure water (v:v:v)). The mice were euthanized on the 4th day after challenge and their lungs were collected. The weight of the mice was recorded daily. The left lung was fixed in tissue fixative solution, embedded, sectionized and stained with H&E to observe the pathological changes of lung tissue. After weighing the right lung, add 400μL PBS into the tube, grind it with a grinding instrument. One part of the grinding tissue was used to determine the virus titer, and the other part was used to determine virus copy number by extracted RNA from the tissue supernatant using viral DNA/RNA extraction Kit (TaKaRa, 9766). The determination of viral titers and subsequent treatment of the RNA obtained were the same as above. Pharmacokinetic study of Mindeudesivir (VV116) in ICR mice, Balb/c mice, and SD rats [1] ICR mice (N = 3 for each group, male) were fasted for 12 h before dosing (only for the oral administration). Mindeudesivir (VV116) dissolved in DMSO-enthanol-PEG300-saline (5/5/40/50, v/v/v/v) was administered intravenously at 5.0 mg/kg, and orally at 25 mg/kg. At 5 min, 0.25, 0.5, 1.0, 2.0, 4.0, 6.0, 8.0, and 24 h post-dosing, blood samples were collected from the jugular vein or the submandibular vein into EDTA-K2 tubes, and immediately mixed with acetonitrile (20 µL blood + 80 μL acetonitrile). The concentrations of analytes in the blood were analyzed by LC-MS/MS. A total of nine Balb/c mice (N = 3 for each group, male) were divided into three groups, and fasted for 12 h before dosing. The three groups received oral dose of Mindeudesivir (VV116) dissolved in 40%PEG400+10% Kolliphor® HS15+50% ultrapure water at 25 mg/kg, 50 mg/kg and 100 mg/kg, respectively. At 5 min, 0.25, 0.5, 1.0, 2.0, 4.0, 6.0, 8.0, and 24 h post-dosing, blood samples were collected from the jugular vein or the submandibular vein into EDTA-K2 tubes, and immediately mixed with acetonitrile (20 µL blood + 80 μL acetonitrile). The concentrations of analytes in the blood were analyzed by LC-MS/MS. SD rats (N = 3 for each group, male) were fasted for 12 h before dosing (only for the oral administration). The test compound (Mindeudesivir (VV116) or VV116-H) was administered intravenously at 5.0 mg/kg dissolved in DMSO-enthanol-PEG300-saline (5/5/40/50, v/v/v/v), and administered orally at 30 mg/kg dissolved in 40%PEG400+10% Kolliphor® HS15+50% ultrapure water. At 5 min, 0.25, 0.5, 1.0, 2.0, 4.0, 6.0, 8.0, and 24 h post-dosing, blood samples were collected from the jugular vein into EDTA-K2 tubes. Serum samples were obtained following general procedures and the concentrations of analytes in the supernatant were analyzed by LC-MS/MS. Tissue distribution study of Mindeudesivir (VV116) in Balb/c mice [1] A total of thirty Balb/c mice were divided into five groups (3 animals/sex/group). VV116 was intragastrically administered at 100 mg/kg dissolved in 40%PEG400+10% Kolliphor® HS15+50%. At 0 (not administered), 0.25, 2, 6, and 24 h post-dosing, the five groups of mice were anesthetized, respectively. Blood samples were collected, and tissues including liver and lung were harvested. Tissue samples were individually homogenized, and blood samples were processed as above. The concentrations of X1 in liver, lung and blood were analyzed by LC-MS/MS. Genetic toxicity assay [1] The Ames test, the rat micronucleus assay, and the chromosome aberration test were conducted according to NMPA and ICH guidelines. The Ames test was conducted to determine the mutagenicity of Mindeudesivir (VV116) using histidine-dependent Salmonella typhimurium (TA97a, TA98, TA100, TA1535) and tryptophan-dependent Escherichia coli (WP2). The experiment was carried out by plate permeating method under the -S9 non-metabolic and +S9 metabolic activation conditions. There were 6 dose groups for Mindeudesivir (VV116) (5, 50, 150, 500, 1500 and 5000 µg/dish under each condition) with the negative control (DMSO) and positive controls (ICR191, 2-nitrofluorene, sodium azide, 2-aminofluorene and methyl methanesulfonate). Under the conditions of -S9 and +S9, the average numbers of revertant colonies in the positive control group of each strain were at least twice that of the negative control group. The numbers of revertant colonies of each strain in all VV116 dose groups were less than twice that of the negative control group, and did not show dose-dependent increase. The result showed that VV116 was not mutagenic to histidine-dependent Salmonella typhimurium and tryptophan-dependent Escherichia coli. The chromosome aberration test was conducted to evaluate whether Mindeudesivir (VV116) had the effect of inducing chromosome damage in Chinese hamster lung (CHL) cells by determining the aberration rate (excluding chromosome gap) under the -S9 and +S9 conditions. CHL cells were exposed to Mindeudesivir (VV116) without S9 for 4 h at the concentrations of 10, 20, 35, 40, 43, 45 and 48 μg/mL (-S9/4h group), or 24 h at the concentrations of 5, 10, 20, 25, 30, 35 and 40 μg/mL (-S9/24h group). In the presence of S9 mix, CHL cells were treated with VV116 for 4 h at the concentrations of 10, 25, 50,100 and 150 μg/mL (+S9/4h group). Meanwhile, negative (DMSO), and positive control groups (Mitomycin C and cyclophosphamide monohydrate) were set up. Based on the cytotoxicity of VV116, three doses of each group were chosen for chromosome aberration analysis. The positive compounds obviously induced chromosome aberrations compared with the negative control. For the -S9/4h group of VV116, the chromosome aberration rates at the concentrations of 20, 35 and 40 µg/mL were 0.0%, 0.3% and 0.0%, respectively; For the -S9/24h group, the rates at the concentrations of 10, 25 and 30 µg/mL were 1.0%, 0.3% and 0.3%, respectively. And for the +S9/4h group, the rates at the concentrations of 20, 50 and 150 µg/mL were 1.3%, 0.7% and 0.3%, respectively. The chromosome aberration rates of all Mindeudesivir (VV116) groups were within the background range, and showed no statistical difference compared with that of the negative control group. The result indicated that VV116 had no effect of inducing chromosome aberration in CHL cells. The micronucleus assay in rats was conducted to evaluate whether Mindeudesivir (VV116) has the effect of inducing any increase of micronucleated polychromatic erythrocytes in rat bone marrow. Groups of male and female SD rats (5 animals/sex/group) received oral doses of VV116 at 0 (vehicle control), 100 (low), 200 (mild) and 500 mg/kg/d (high) for 14 days. The animals were sacrificed within 24 h after the last dose. Bone marrow smears were prepared for examining the ratio of polychromatic erythrocyte/(polychromatic erythrocyte + normochromatic erythrocyte) (PCE/(PCE + NCE)) and the micronucleus rate of polychromatic erythrocytes (MnPCE/PCE). The result showed that the PCE/(PCE + NCE) ratios of the female animals of the vehicle group, the low, the mild, and the high dose VV116 group were 0.65, 0.57, 0.58 and 0.58, respectively. For the male animals, the ratios were 0.62, 0.64, 0.66 and 0.60, respectively. VV116 did not show obvious bone marrow toxicity in rats. The assay was valid as the average micronucleus rates were 1.4‰ and 0.7‰ for the female and male rats in the vehicle group, respectively, which were within the historical range. The micronucleus rates of the female animals of the three VV116 groups were 1.2‰, 1.0‰ and 0.7‰, respectively, and for the male animals, the rates were 0.3‰, 0.7‰ and 0.3‰, respectively. There was no effect of any dose of VV116 on the micronucleus rate compared to the negative control. VV116 did not have the effect of inducing the increase of micronucleated polychromatic erythrocytes in rat bone marrow up to 500 mg/kg/d for 14 days. Toxicokinetics of Mindeudesivir (VV116) in SD rats [1] Groups of male and female SD rats (4 animals/sex/group) received repeated oral doses of Mindeudesivir (VV116) (dissolved in 40%PEG400+10% Kolliphor® HS15+50% ultrapure water) at 100 (low), 200 (mild) and 500 mg/kg/d (high) for 14 days. At day 1 and day 14, blood samples were collected from the jugular vein into EDTA-K2 tubes at various time points post-dose. Plasma samples were obtained following general procedures and the concentrations of analytes in the samples were analyzed by LC-MS/MS. |
| 药代性质 (ADME/PK) |
VV116 (JT001) is an oral drug candidate of nucleoside analog against SARS-CoV-2. The purpose of the three phase I studies was to evaluate the safety, tolerability, and pharmacokinetics of single and multiple ascending oral doses of VV116 in healthy subjects, as well as the effect of food on the pharmacokinetics and safety of VV116. Three studies were launched sequentially: Study 1 (single ascending-dose study, SAD), Study 2 (multiple ascending-dose study, MAD), and Study 3 (food-effect study, FE). A total of 86 healthy subjects were enrolled in the studies. VV116 tablets or placebo were administered per protocol requirements. Blood samples were collected at the scheduled time points for pharmacokinetic analysis. 116-N1, the metabolite of VV116, was detected in plasma and calculated for the PK parameters. In SAD, AUC and Cmax increased in an approximately dose-proportional manner in the dose range of 25-800 mg. T1/2 was within 4.80-6.95 h. In MAD, the accumulation ratio for Cmax and AUC indicated a slight accumulation upon repeated dosing of VV116. In FE, the standard meal had no effect on Cmax and AUC of VV116. No serious adverse event occurred in the studies, and no subject withdrew from the studies due to adverse events. Thus, VV116 exhibited satisfactory safety and tolerability in healthy subjects, which supports the continued investigation of VV116 in patients with COVID-19.[2]
The PK study in Balb/c mice showed that Mindeudesivir (VV116) had a linear PK profile in doses of 25 to 100 mg/kg (Fig. 1c, supplementary Table S6). Because of the first-pass metabolism of the esterase-sensitive prodrug, VV116 was not detected in mouse plasma even at 100 mg/kg. Following oral administration, the blood concentration of the parent nucleoside X1 quickly reached Cmax within 0.5 h, and at the dose of 25 mg/kg, the mean Cmax reached 5360 ng/ml (18.4 µM, Fig. 1c, supplementary Table S6, S7), which was much higher than the EC90 value in vitro. X1 had a short elimination half-life (2.3–4.25 h, supplementary Table S6), which supported a twice-daily dosing regimen. The ester prodrug form of VV116 was designed not only for improving oral adsorption but to circumvent the liver-targeting issue of the nucleoside phosphoramidate prodrugs. The preclinical tissue distribution study revealed that X1 was widely distributed in SD rat tissues,5 and a favorable distribution of X1 was also observed in Balb/c mice with the concentration of X1 in the lung being about half of that in the liver (Fig. 1d, supplementary Table S8). With respect to the therapeutic window of VV116, the 14-day repeated dose oral toxicity study in rats revealed a NOAEL (No-Observed-Adverse-Effect-Level) of 200 mg/kg, at which the AUC0–t of X1 reached a value of 85151 ng h/ml (Supplementary Table S9), ~3.5-folds of that at the dose of 50 mg/kg in mice.[1] |
| 毒性/毒理 (Toxicokinetics/TK) |
Safety [2]
No deaths, serious adverse event (SAE), AEs of Grade 3 or above, or AEs leading to drug discontinuation/interruption were reported throughout the three studies. All AEs were recovered without any treatment or intervention. Study 1: single ascending-dose study The number (incidence) of subjects experiencing AEs for 25, 200, 400, 800, and 1200 mg dose group and placebo group was 2 (50%), 3 (50%), 3 (50%), 3 (50%), 0 (0), and 5 (50%), respectively (Table 7). No relation with dose was observed for the AE occurrence. The incidence of AE for subjects administered Mindeudesivir (VV116) in total was lower than those administered placebo (39.3% vs. 50%) in a single ascending-dose study. The severity of AEs was CTCAE Grade 1 with the exception of one case of Grade 2 neutropenia. The dose-escalation termination criteria were not met during dose escalation. The most common drug-related AEs were sinus bradycardia, shortened electrocardiogram PR, and increased blood bilirubin. Study 2: multiple ascending-dose study The number (incidence) of subjects experiencing AEs for 200 mg, 400 mg, 600 mg dose group and placebo group was 3 (33.3%), 5 (55.6%), 6 (66.7%), and 5 (55.6%), respectively (Table 8). The incidence of AE for subjects administered Mindeudesivir (VV116) in total was comparable with those administered placeboes (51.9% vs. 55.6%). AE occurrence was detected to be related to dose. Besides one subject in the placebo group experienced three cases of Grade 2 nausea, the severity of AEs was generally mild (CTCAE Grade 1). The most common drug-related AEs were increased blood uric acid, dry mouth, presence of crystal urine, and nausea. Three cases had increased transaminases (increased alanine aminotransferase, increased aspartate aminotransferase, and increased gamma-glutamyltransferase) with Grade 1 observed in two subjects of 400 mg dose group. Transaminase increase was transient, and recovered spontaneously. Study 3: food-effect study The number (incidence) of subjects experiencing AEs under fasting condition, fed condition with a standard meal, fed condition with a high-fat meal was 0 (0), 2 (16.7%), and 4 (33.3%). Two subjects under fed condition with a standard meal were observed atrioventricular block with first degree, while four subjects under the fed condition with a high-fat meal experienced positive results in urine bacterial test, presence of crystal urine, increase in blood pressure, and atrioventricular block with first degree. All AEs were CTCAE Grade 1 in severity. Other safety assessments Only one subject in 400 mg dose group of Study 3 experienced mild increase in transient blood thyroid-stimulating hormone, which recovered spontaneously without any treatment. No clinically significant abnormality was discovered in sex hormone test, ophthalmological examination, and thyroid B ultrasound test. |
| 参考文献 |
|
| 其他信息 |
Nucleoside antiviral agents have a high genetic barrier to resistance since they target the highly conserved catalytic center of viral polymerase, and VV116 has been found to be effective against different SARS-CoV-2 variants. The favorable PK properties and good safety profile make it to be a very promising oral antiviral for treating COVID-19. Herein, the in vivo efficacy study also provided strong evidence for potential therapeutic usage of VV116 against RSV infection. The clinical studies of VV116 should be considered to mitigate RSV infection.[1]
Mindeudesivir (VV116) is a prodrug of nucleoside analog, intended for the treatment of COVID-19. RDV is the first FDA-approved drug for the treatment of COVID-19, which is also a nucleoside analog. Compared with RDV, VV116 exhibits better in vitro antiviral activity and selectivity. In addition, VV116 could be administered orally and has favorable oral bioavailability, that is more convenient for COVID-19 patients than intravenous administration of RDV. [2] Mindeudesivir (VV116) was hydrolyzed rapidly to its metabolite 116-N1 after oral administration. 116-N1, instead of the prototype drug VV116, was detected in plasma, and calculated for the PK parameters. Peak plasma 116-N1 concentration reached quickly after oral administration (median Tmax 1.00–2.50 h). In the single ascending-dose study, AUC and Cmax increased in an approximately dose-proportional manner in the dose range of 25–800 mg. However, the parameters did not show significant change with dose escalation from 800 to 1200 mg (AUC0-t: 25886 vs. 28057 h·ng/mL; Cmax: 2796 vs. 3086 ng/mL), indicating the probability of drug absorption saturation. Drug solubility is an important factor affecting the drug absorption and maximum drug absorption occurs when the drug has maximum concentration (saturation solubility) at the site of absorption. It was suspected that limited solubility of VV116 might be the reason for drug absorption saturation. The fractional excretion of 116-N1 in urine was 53.6% in 0–72 h after administration, while that of 116-N1 and VV116 in feces was 5.25%, which indicated that VV116 was principally excreted through kidney in the form of metabolite 116-N1. [2] The mean t1/2 of Mindeudesivir (VV116) was 4.80–6.95 h in the single ascending-dose study, suggesting twice-daily dosing in the clinical treatment. Thereby, continuous twice-daily dosing (12 h apart) for 5.5 days (days 1–6) was adopted in the multiple ascending-dose study. The accumulation ratio of AUC parameters and Cmax indicated a slight accumulation of VV116 after continuous dosing. The trough concentrations of 116-N1 following multiple administration of 200 mg at day 5 and day 6 were within 242–345 ng/mL (Table 5), which were above the EC90 (186.5 ng/mL) of 116-N1 against the omicron variant in a preclinical anti-SARS-CoV-2 assay. Therefore, the dosage regimen of 200 mg BID and above can continuously maintain the effective antiviral concentration, and is recommended for subsequent clinical studies in patients with COVID-19. [2] The median Tmax under fasting, standard meal and high-fat meal condition was 1.50, 3.00, and 2.50 h, respectively, indicating that fed condition could prolong the time to the peak. Compared with fasting condition, the GMR (90% CIs) of Cmax under fed condition with both standard meal and high-fat meal was within the equivalent range 80%–125%; the GMR (90% CIs) of AUC for standard meal was also within the range 80%–125%, however for high-fat meal, AUC0-t and AUC0-∞ slightly increased by 26.32% and 24.67%, respectively. Since food intake has no effect on Cmax of Mindeudesivir (VV116), while high-fat meal slightly increases AUC, it is recommended that VV116 could be taken under fasting condition or fed condition with regular meal in the treatment of COVID-19. [2] In the single ascending-dose study, there was no apparent dose-related trend, with a greater proportion of subjects reporting AEs following administration of placebo (50.0%) than following administration of Mindeudesivir (VV116) (39.3%). The severity of AEs was CTCAE Grade 1 with the exception for one case of Grade 2 neutropenia. In the multiple ascending-dose study, the incidence of AEs in the VV116 group was comparable with that in the placebo group (51.9% vs. 55.6%). AE occurrence was slightly dose-related. Only 1 subject in 400 mg dose group reported one case of increased alanine aminotransferase and increased aspartate aminotransferase, respectively. All AEs in subjects administered VV116 were Grade 1 in severity, and were recovered without any treatment. No serious adverse event occurred throughout the study, and no subject withdrew from the study due to AE. In the preclinical animal toxicology study, it was discovered that VV116 might have toxicity on eyes, thyroid, and gonads. In our studies, ophthalmology examination, thyroid function, thyroid B ultrasound, and sex hormone tests were performed on healthy subjects before and after VV116 administration. No obvious toxicity was observed in the above organs. Overall, VV116 demonstrated satisfactory safety profiles in healthy subjects throughout the three studies. [2] Hepatotoxicity is the primary adverse drug reaction (ADR) of RDV, manifested as transaminase elevation. In phase I clinical study (Study GS-US-399-5505), subjects received one loading dose of 200 mg RDV followed by 100 mg for up to 9 days, transient ALT elevation of Grade 1 or 2 was observed in 9 of 20 subjects (45%). Transaminase elevation has also been reported as the most frequent ADR in patients with COVID-19 who received RDV. In the multiple ascending-dose study of Mindeudesivir (VV116), only 1 of 27 subjects (3.7%) experienced transient ALT elevation of Grade 1, which recovered spontaneously after VV116 termination. This can be explained by the high targeting capability of RDV to the liver and its liver/blood concentration ratio is about 21 times that of VV116. The liver/blood concentration ratio of RDV (calculated by equivalents 14C-GS-5734) after a single intravenous administration of 10 mg/kg [14C]RDV at 4 h was 57.8, while the ratio of the VV116 (calculated by major metabolite 116-N1) after a single oral dose of 30 mg/kg VV116 to rat at 2 h was only 2.8. Despite the lower risk of hepatotoxicity of VV116 compared to RDV, monitoring for the hepatic function will continue in the subsequent phase II study of VV116 in COVID-19 patients. [2] Conclusions [2] Mindeudesivir (VV116) exhibited satisfactory safety and tolerability in healthy subjects. Peak plasma drug concentration of 116-N1 reached quickly after oral administration of VV116 (median Tmax 1.00–2.50 h). AUC and Cmax increased in an approximately dose-proportional manner in the dose range of 25–800 mg, while drug absorption saturation was probably achieved at the dose of 800 mg. Standard meal had no effect on Cmax and AUC of VV116. Effective antiviral concentration was achieved at dose levels between 200 and 600 mg BID following multiple administration. [2] In conclusion, the safety data and PK profile from these studies support the continued investigation of VV116 in patients with COVID-19. The COVID-19 pandemic remains uncontrolled despite the rapid rollout of safe and effective SARS-CoV-2 vaccines, underscoring the need to develop highly effective antivirals. In the setting of waning immunity from infection and vaccination, breakthrough infections are becoming increasingly common and treatment options remain limited. Additionally, the emergence of SARS-CoV-2 variants of concern with their potential to escape therapeutic monoclonal antibodies emphasizes the need to develop second-generation oral antivirals targeting highly conserved viral proteins that can be rapidly deployed to outpatients. Here, we demonstrate the in vitro antiviral activity and in vivo therapeutic efficacy of GS-621763, an orally bioavailable prodrug of GS-441524, the parental nucleoside of remdesivir, which targets the highly conserved RNA-dependent RNA polymerase. GS-621763 exhibited significant antiviral activity in lung cell lines and two different human primary lung cell culture systems. The dose-proportional pharmacokinetic profile observed after oral administration of GS-621763 translated to dose-dependent antiviral activity in mice infected with SARS-CoV-2. Therapeutic GS-621763 significantly reduced viral load, lung pathology, and improved pulmonary function in COVID-19 mouse model. A direct comparison of GS-621763 with molnupiravir, an oral nucleoside analog antiviral currently in human clinical trial, proved both drugs to be similarly efficacious. These data demonstrate that therapy with oral prodrugs of remdesivir can significantly improve outcomes in SARS-CoV-2 infected mice. Thus, GS-621763 supports the exploration of GS-441524 oral prodrugs for the treatment of COVID-19 in humans. [4] |
| 分子式 |
C24H30DN5O7
|
|---|---|
| 分子量 |
502.54
|
| 精确质量 |
502.228
|
| 元素分析 |
C, 57.36; H, 6.42; N, 13.94; O, 22.29
|
| CAS号 |
2647442-33-7
|
| 相关CAS号 |
GS-621763;2647442-13-3; 2647442-33-7; 2779498-79-0 (HBr)
|
| PubChem CID |
163358784
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
2.3
|
| tPSA |
168
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
11
|
| 可旋转键数目(RBC) |
11
|
| 重原子数目 |
36
|
| 分子复杂度/Complexity |
887
|
| 定义原子立体中心数目 |
4
|
| SMILES |
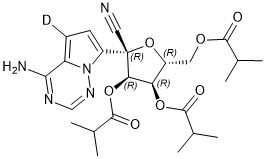
[2H]C1=C2C(=NC=NN2C(=C1)[C@]3([C@@H]([C@@H]([C@H](O3)COC(=O)C(C)C)OC(=O)C(C)C)OC(=O)C(C)C)C#N)N
|
| InChi Key |
RVSSLHFYCSUAHY-QXMJNOOVSA-N
|
| InChi Code |
InChI=1S/C24H31N5O7/c1-12(2)21(30)33-9-16-18(34-22(31)13(3)4)19(35-23(32)14(5)6)24(10-25,36-16)17-8-7-15-20(26)27-11-28-29(15)17/h7-8,11-14,16,18-19H,9H2,1-6H3,(H2,26,27,28)/t16-,18-,19-,24+/m1/s1/i7D
|
| 化学名 |
(2R,3R,4R,5R)-2-(4-amino-5-deuteropyrrolo[2,1-f][1,2,4]triazin-7-yl)-2-cyano-5-((isobutyryloxy)methyl)tetrahydrofuran-3,4-diyl bis(2-methylpropanoate)
|
| 别名 |
VV116; VV 116; VV-116; JT001; JT-001; JT 001;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL ( ~198.98 mM )
Ethanol : ~100 mg/mL
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9899 mL | 9.9495 mL | 19.8989 mL | |
| 5 mM | 0.3980 mL | 1.9899 mL | 3.9798 mL | |
| 10 mM | 0.1990 mL | 0.9949 mL | 1.9899 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT06206720 | RECRUITING | Drug:Deuremidevir Hydrobromide for Suspension Drug:Placebo |
Respiratory Syncytial Virus Infection | Vigonvita Life Sciences | 2024-01-31 | Phase 2 |
| NCT05201690 | COMPLETED | Drug:VV116 200 mg Group Drug:VV116 400mg Group Drug:VV116 600mg Group |
Healthy Subjects | Vigonvita Life Sciences | 2021-12-14 | Phase 1 |
| NCT05227768 | COMPLETED | Drug:VV116 25mg Group Drug:VV116 200mg Group Drug:VV116 400mg Group |
Healthy Subjects | Vigonvita Life Sciences | 2021-11-11 | Phase 1 |
| NCT05279235 | TERMINATED | Drug:JT001 Drug:JT001 placebo Drug:Favipiravir Drug:Favipiravir placebo |
Moderate to Severe COVID-19 | Shanghai Vinnerna Biosciences Co.,Ltd. |
2022-03-14 | Phase 3 |
| NCT05355077 | WITHDRAWN | Drug:JT001 200mg Bid Drug:JT001 400mg Bid Drug:JT001 600mg Bid |
Healthy Subjects | Shanghai Vinnerna Biosciences Co.,Ltd. | 2022-05-02 | Phase 1 |