| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg | |||
| Other Sizes |
| 靶点 |
p110α (IC50 = 32 nM); p110α E545K (IC50 = 30 nM); p110α H1047R (IC50 = 43 nM); p110γ (IC50 = 3480 nM); PI3K-C2β (IC50 = 462 nM); PI4Kβ (IC50 = 236 nM)
|
|---|---|
| 体外研究 (In Vitro) |
p110α 的致癌形式,如 p110α E545K 和 p110α H1047R,也被 A66 有效抑制,IC50 值分别为 30 nM 和 43 nM。与其他 I 类 PI3K 同工型相比,A66 对 p110α 的选择性大于 100 倍,而 PIK-75 则相反。在 II 类 PI3K、III 类 PI3K 和 PI4K 中,A66 仅与 II 类 PI3K PI3KC2β 和 PI4K 的 PI4Kβ 亚型表现出有限的交叉反应性,IC50 分别为 462 nM 和 236 nM。相关激酶 DNA-PK 和 mTOR 以及其他脂质激酶不受 A66 抑制。与 PIK-75 相比,在 10 μM 浓度下针对两个相当大的组(110 种蛋白激酶和 318 种激酶)进行测试时,A66 具有更高水平的特异性。在一些 PIK3CA 具有 H1047R 突变且 p110 和 Ia 类 PI3K 活性水平较高的细胞系中,通过 A66 治疗抑制 p110α 足以阻断向 Akt/PKB 发出的胰岛素信号传导。 [1]高度转化的 p85 iSH2 突变体 KS459delN、DKRMN-S560del 和 K379E 在接受 0.7 μM A66 后,病灶形成减少 75–80%,并且所有 p85α iSH2 突变体都减少了 T308 上的 Akt 磷酸化。 [2]
抑制剂特异性[1] 首先对A66进行了表征,并证实它是野生型和致癌型p110α的强效抑制剂,但不是其他I类PI3K亚型(表1)。发现A66对p110α的选择性比PIK-75高得多。鉴于II类PI3Ks、III类PI3K[38]和PI4Ks(磷酸肌醇4-激酶)在生长因子信号传导中的重要作用,我们还评估了A66对这些的活性,发现与II类PI3K PI3K-C2β和PI4K的PI4Kβ亚型存在一些有限的交叉反应(表2)。对其他脂质激酶或相关激酶DNA-PK和mTOR没有抑制作用(表2)。我们还测试了10μM A66效应对两组110种蛋白激酶的抑制作用(补充图S1http://www.BiochemJ.org/bj/438/bj4380053add.htm)318种激酶(补充图S2http://www.BiochemJ.org/bj/438/bj4380053add.htm). 这些表明A66是p110α的一种非常特异的抑制剂,而PIK-75,即之前描述为p110α选择性抑制剂的化合物,在该浓度下抑制了大量蛋白激酶(补充图S1)。我们使用HTRF测定法生成的TGX-221和IC87114数据与之前使用其他测定方法的研究一致,并证实这些分别是p110β和p110δ的高选择性抑制剂(表1),尽管TGX-221在较高浓度下会与p110δ发生交叉反应。我们进一步报告说,这些抑制剂对110种蛋白激酶没有任何重大影响(补充图1)。 特异性抑制p110α对细胞功能的影响[1] 为了研究p110α在调节PI3K依赖性信号通路近端元件中的作用,我们通过Ser473和Thr308的磷酸化评估了不同浓度的A66S形式在一系列细胞系中急性阻断Akt/PKB激活的能力(图3)。通过对整个PKB进行重新编程来控制负载(见补充图S3http://www.BiochemJ.org/bj/438/bj4380053add.htm). 我们发现,在所有测试的细胞系中,Ser473和Thr308的磷酸化对LY294002都很敏感,这意味着激活Akt/PKB需要I类PI3K活性。然而,我们发现抑制Ser473和Thr308磷酸化所需的A66S形式的量遵循两种不同的模式,要么对A66 S形式在与通过p110α起作用的浓度一致的浓度下的抑制敏感,要么具有抗性。敏感细胞系最明显的特征是它们在PIK3CA中携带H1047R突变,而所有其他细胞系都是抗性的。作为对照,我们测试了A66 R形式的作用,发现它不能抑制Akt/PKB的磷酸化(见补充图S4http://www.BiochemJ.org/bj/438/bj4380053add.htm). |
| 体内研究 (In Vivo) |
在体内,给药后 1 小时和 6 小时,单剂量 100 mg/kg 的 A66 导致 Akt/PKB 和 p70 S6 激酶的磷酸化显着降低,但 ERK 的磷酸化没有显着降低。与众所周知的泛 PI3K 抑制剂 BEZ-235 相比,A66 导致 SK-OV-3 异种移植肿瘤的生长速度显着减慢,每日一次 100 mg/kg 剂量给药时,平均 TGI 分别为 45.9% 和 29.9% (QD) 持续 21 天或 75 mg/kg 每天两次 (BID) 持续 16 天。 A66 的 QD 给药虽然导致 U87MG 异种移植模型中的肿瘤体积没有显着减少,但也导致 HCT-116 异种移植模型中的肿瘤体积显着减少,TGI 为 77.2%。 [1]在雄性 CD1 小鼠中,给予 10 mg/kg A66 会导致 ITT(胰岛素耐量试验)和 GTT(葡萄糖耐量试验)显着受损,并且在 PTT(丙酮酸耐量试验)期间葡萄糖产生增加,几乎与泛 PI3K 抑制剂引起的症状一样严重。 [3]
根据药代动力学和药效学研究结果,在肿瘤疗效研究中,每天一次以100mg/kg体重给药A66S,持续21天,或每天两次以75mg/kg体重给药,持续16天。两种给药策略都显著延迟了SK-OV-3异种移植物肿瘤的生长,甚至超过了公认的泛PI3K抑制剂BEZ-235诱导的生长(图7A)。在给药的最后一天,A66 S型的平均TGI为对照组的45.9%(QD;P<0.05)和对照组的29.9%(BID;P<0.01)(表4)。QDA66S在该异种移植物模型中耐受良好,体重减轻最小;然而,BID治疗与中度体重减轻和两例死亡有关,尽管尚不清楚死亡是由于药物毒性还是其他原因,因为这些小鼠没有表现出明显的体重减轻(图7B)。相比之下,BEZ-235诱导的肿瘤生长没有显著减少,甚至耐受性更低,体重适度减轻,4人死亡。HCT-116异种移植物模型中每天一次给予A66S也诱导了肿瘤体积的显著减少,给药结束时TGI为对照组的77.2%(P<0.01),但在U87MG异种移植模型中,肿瘤体积没有显著减少(表4和补充图S5http://www.BiochemJ.org/bj/438/bj4380053add.htm). 相比之下,BEZ-235显著降低了U87MG肿瘤的生长(TGI=对照组的61.1%;P<0.05),但对HCT-116肿瘤没有影响。尽管SK-OV-3研究中BEZ-235的剂量水平相同,但U87MG模型和HCT-116模型中的药物耐受性良好,在HCT-116模式中,由于对照组治疗的小鼠体重中度减轻,使用了较低剂量(10mg/kg体重)的BEZ-235。[1] 本研究首次研究了选择性p110α抑制剂(A66)对体内葡萄糖代谢的影响。我们发现A66会损害体内胰岛素作用的所有指标,几乎与pan-PI3K抑制剂的水平相同。这提供了强有力的药理学证据,表明p110α是急性调节葡萄糖代谢途径中最重要的亚型,PI3K亚型之间的功能冗余不太可能是体内调节葡萄糖代谢主要途径的主要特征。A66对葡萄糖代谢的影响是p110α激酶死亡形式全局表达杂合小鼠的表型。然而,尽管A66在全球范围内抑制p110α,但本研究的结果也与仅在肝脏中急性或慢性缺失Pik3ca基因的小鼠的结果非常相似。结合我们的PTT结果,这表明p110α在调节胰岛素对葡萄糖代谢的影响方面的主要作用位点在肝脏[3]。 |
| 酶活实验 |
PI3K(人类)HTRF 检测用于计算 IC50 值。 Invitrogen 提供 p85α/p110δ 。所有其他亚型都是通过将全长人 p85α 与适当的全长人催化亚基结合而现场制备的,该亚基在 N 末端标有组氨酸标签以方便纯化。 PI3K 的使用浓度在其 EC65 和 EC80 值之间进行滴定。使用 p85α N-SH2(N-Src 同源 2)结构域的抗体,测量免疫沉淀物中的 PI3K 活性。国家蛋白激酶分析中心和 Invitrogen 药物发现服务中心对其他脂质激酶和蛋白激酶进行检测[1]。
|
| 细胞实验 |
细胞培养和转染。[2]
受精鸡蛋(白色Leghorn)购自Charles River育种实验室。如前所述制备和培养原代CEF。对于转染,细胞在含有5.8%补铁FCS和1%L-谷氨酰胺-青霉素-链霉素溶液的F-10中以80%的融合率铺板。第二天,使用二甲亚砜/聚异戊二烯法用RCAS载体转染CEF。在血清存在下传代两次后,收获细胞进行进一步分析。 HEK 293-T细胞在添加了10%FCS和1%L-谷氨酰胺-青霉素-链霉素溶液的DMEM中培养。根据制造商的方案,使用脂质体PLUS进行输血。将70%融合的MP6板中的HEK293T细胞用Opti-MEM培养基洗涤一次,并在0.8mL Opti-MEM中孵育。在室温下将总共1μg质粒DNA与0.1mL Opti-MEM和2μL Lipofectamine PLUS混合15分钟。将含有6μL Lipofectamine的Opti-MEM(0.1 mL)加入DNA-PLUS混合物中,并在室温下孵育15分钟。将DNA、PLUS和Lipofectamine的混合物加入细胞中并孵育过夜。第二天,将培养基换成含有10%FCS和1%L-谷氨酰胺-青霉素-链霉素溶液的DMEM。转染40小时后,收集细胞,裂解并分析特定蛋白质。 焦点分析。[2] 如前所述,使用感染性逆转录病毒载体进行了重点检测。使用二甲亚砜/聚异戊二烯法用适当的RCAS构建体转染CEF,每隔一天用营养琼脂覆盖2至3周,直至观察到焦点形成。用结晶紫对平板进行染色,并计数转化细胞的焦点。为了检测不同化合物对焦点形成的抑制作用,将10μM LY294002、100 nM NVP-BEZ-235、2 nM雷帕霉素、5μM ZK-93、250 nM TGX221、5μM IC87114或5μM AS604850添加到每个覆盖层的营养琼脂中。 细胞增殖。[2] 转染后,将CEF分成含有F-10的增殖试验培养基,其中添加了2%的FCS和1%的鸡血清。在第二次分裂时,将细胞以每孔4000个细胞的速度接种到96孔板中。在接种后第1至5天,CEF在37°C的增殖试验培养基中与10μg/mL的Resazurin Na一起孵育4小时。在560nm的激发波长和590nm的发射下测定荧光。 Western Blot和免疫沉淀。[2] 如前所述进行蛋白质印迹,但稍作修改。细胞在改良的Nonidet P-40裂解缓冲液中裂解(20 mM Tris-Cl、150 mM NaCl、1 mM MgCl2、1%Nonident P-40和10%甘油,含1 mM PMSF、1 mM DTT、50 mM NaF、1 mmol Na3VO4、50 mMβ-甘油磷酸盐和蛋白酶抑制剂混合物)。在4°C下以18000×g离心10分钟后,测定上清液的蛋白质浓度。为了检查抑制剂信号传导,在收集之前,在含血清的条件下用250 nM TGX-221或5μM IC87114处理细胞2小时。为了进行免疫沉淀,将含有40μg蛋白质的细胞裂解物与抗FLAG M2琼脂糖在4°C下孵育过夜。琼脂糖珠用裂解缓冲液洗涤四次,加热至95°C,然后在SDS-PAGE凝胶上分离。转移到Immobilon P膜后,这些膜在室温下用含0.1%吐温-20(TBS-T)的Tris缓冲盐水中的5%BSA封闭2小时,然后用1:2000的抗FLAG或1:1000的抗p110α或抗p110β一抗稀释液孵育过夜。将膜在TBS-T中洗涤三次,并在室温下与过氧化物酶偶联的山羊抗小鼠或山羊抗兔抗体在5%BSA/TBS-T溶液中孵育1小时。反应带通过SuperSignal West Pico化学发光底物可视化。 对于蛋白质印迹,在SDS-PAGE凝胶上分离含有10μg总蛋白的细胞裂解物,并将其转移到Immobilon P膜上。将膜与1:1000稀释的针对p85、pAkt(T308)、Akt、p4E-BP、4E-BP和β-actin的一抗一起孵育。如上所述,开发了蛋白质印迹。抗FLAG抗体(FLAG-M2 F3165)购自xxx。从xyz获得抗p110α抗体(#4255)、抗p110β抗体(#3011S)、抗p85抗体(#4292)、抗Akt(#2967)、抗磷酸化Akt(Thr-308)(#9275S)、抗4E-BP1(#9452)和抗磷酸化4E-BPl(Ser-65)(#9451S)抗体。 |
| 动物实验 |
Mice: Subcutaneous inoculation of 5×106 U87MG, SK-OV-3, or HCT-116 cells in PBS is performed on the right flank of age-matched, pathogen-free Rag1-/- or NIH-III mice. Based on the formula (L×w2)×π/6 (where L is the longest tumour diameter and w is the perpendicular diameter), tumour volume (mm3) is calculated using the tumor diameter as measured by electronic calipers. While BEZ-235 is administered in 10% ethanol, A66 is given in 20% 2-hydroxypropyl-β-cyclodextrin in water. The A66 dosing vehicle is given to control mice only. The drugs are administered intraperitoneally at a dose volume of 10 mL/kg of body weight as the free base equivalent. When tumors have grown to a diameter of about 8 to 9 mm, mice are given a single dose of A66 or the control substance for tumor pharmacodynamic studies. The tumors are removed, biopulverized, and the protein concentration is measured before the animals are killed 1 or 6 hours after the last dose.
Xenograft methods [1] Age-matched specific pathogen-free Rag1−/− or NIH-III mice were subcutaneously inoculated on the right flank with 5×106 U87MG, SK-OV-3 or HCT-116 cells in PBS. Tumour diameter as measured by electronic calipers was used to calculate tumour volume (mm3) based on the formula (L×w2)×π/6 (where L=longest tumour diameter and w=perpendicular diameter). A66 was administered in 20% 2-hydroxypropyl-β-cyclodextrin in water, whereas BEZ-235 was administered in 10% ethanol. Control mice were administered the A66 dosing vehicle alone. The drugs were dosed by intraperitoneal injection as the free base equivalent at a dosing volume of 10 ml/kg of body weight. For tumour pharmacodynamic studies, mice were administered a single dose of A66 or the control vehicle when tumours reached approximately 8–9 mm in diameter. Animals were killed 1 or 6 h after dosing and the tumours were removed, biopulverized and assayed for protein concentration. For antitumour efficacy studies, dosing began when tumours were well established, averaging approximately 7 mm in diameter. Doses were administered once daily (QD) or twice daily (BID) with injections separated by a minimum of approximately 8 h. Different dosing schedules were used for the three xenograft models depending on the rate of tumour growth and the body weight tolerance of control mice. Animals were dosed daily for 21 days or twice daily for 16 days (SK-OV-3), daily for 14 days (U87MG) and daily for 7 days (HCT-116). Animals were monitored daily for any signs of emerging toxicity and body weight was recorded. Mice were killed if they developed moderate signs of toxicity or if body weight loss exceeded 20% of starting weight. TGI (tumour growth inhibition) was calculated on the final day of dosing by determining the relative tumour size of drug-treated mice as a percentage of the average relative tumour size of control mice. The statistical significance of TGI values was determined by one-way ANOVA with Bonferroni multiple comparison analysis using GraphPad Prism 5.02. GTT, ITT and PTT [3] GTTs, ITTs and PTTs, as well as determinations of insulin levels, were performed as described previously, except that male CD1 mice were used instead of rats. For GTTs and PTTs the mice were starved overnight and for the ITT food was withdrawn 2 h prior to the start of the experiments. Drugs were dosed intraperitoneally 1 h after the end of the dark cycle and 1 h prior to the intraperitoneal dosing with glucose or pyruvate (2 g/kg of body mass) or insulin (0.75 unit/kg of body mass). Metabolic cage studies [3] Oxymax/CLAMS (Columbus Instruments) was used to quantify oxygen consumption (V̇O2), CO2 production (V̇CO2), BMR (basal metabolic rate), food intake, water intake and animal movement as described previously. BMR was expressed as a function of lean body mass as recommended in a previous study. All data were normalized to total lean mass using the EchoMRI-100 quantitative magnetic resonance system as described previously. Animals were acclimatized for 24 h in cages and the data were collected over the following 24 h. Analysis of drug levels [3] Pharmacological kinetics studies were undertaken in fed CD1 male mice (30 g body mass). Animals were administered with the stated PI3K inhibitors via oral gavage or intraperitoneal injection, and terminal blood samples were collected in EDTA blood collection tubes at 15 min, and 1, 2, 4, 6 and 24 h post-drug exposure. All drugs were dissolved in DMSO. Blood was centrifuged (2000 g for 10 min and 4°C) and plasma isolated for drug quantification. Drug quantification was undertaken using LC-MS/MS (liquid chromatography tandem MS). Briefly, 300 μl of 100% methanol was added to 100 μl of plasma. The samples were gently mixed and centrifuged (2000 g for 10 min and 4°C). The supernatant was removed and 50 μl was added into vials for LC-MS/MS. The ion-source type was ESI (electrospray ionization) with the following conditions: spray voltage (5500 V), sheath gas pressure (50 units), ion sweep gas pressure (0.0 unit), auxillary gas pressure (2 units), capillary temperature [370°C and the capillary offset at 35 V. HPLC kinetex columns were used (100 mm × 3 mm, 2.6u C18(2)-HST; Phenomenex]. The run method was isocratic 10% (0.1% formic acid in water) and 90% methanol. The flow rate was 0.2 ml/min. Retention times were 2.64 min (PI-103), 2.76 min (TGX221) and 2.35 min (IC87114). Unknown concentrations were determined from the standard curve and internal standard. |
| 药代性质 (ADME/PK) |
First, we determined the optimal dosing strategy for xenograft studies by investigating the drug pharmacokinetics after a dose of 10 mg/kg of body weight by intraperitoneal injection in CD-1 mice. Despite a short half-life of only 0.42 h, the large Cmax (8247 nM) of A66 S that was reached 30 min after dosing ensured that the AUC0-inf (area under the curve from zero time to infinity) (6809 nM·h) was similar to that of BEZ-235 (7333 nM·h), which has a longer half-life of 2.73 h (Table 3). Furthermore, we tested the effect of the A66 S form on SK-OV-3 tumour tissue in vivo using a single dose of 100 mg/kg of body weight to determine whether a long-lasting effect of the drug could be achieved on target tissues (Figure 6). These studies show that A66 S causes a profound reduction in the phosphorylation of Akt/PKB and p70 S6 kinase, but not of ERK (extracellular-signal-regulated kinase), at both 1 and 6 h after dosing (Figure 6). This is consistent with A66 S having a full inhibitory effect on PI3K signalling in the tumours during this time. In the present study, levels of A66 S in plasma were determined to be 21.1±1.2 μM and 9.1±1.1 μM at 1 and 6 h after drug injection, whereas levels of A66 S in the tumour were 22.7±2.1 μM and 16.0±1.3 μM at the same time points. Thus, the retention of drug in the tumour is likely to explain the persistence of the inhibitory effect. [1]
Pharmacokinetic methods [1] Age-matched specific pathogen-free male CD-1 mice were administered a single dose of A66 (10 mg/kg of body weight) in 20% 2-hydroxypropyl-β-cyclodextrin in water or BEZ-235 in 15% (v/v) DMSO, 20% (v/v) 0.1 M HCl, 0.7% Tween 20 and 64.3% (v/v) saline. Mice were killed at five or six time points after dosing (n=3/time point) and blood was removed by cardiac puncture into EDTA collection tubes. Blood samples were centrifuged for 10 min at 6000 rev./min at 20 °C and the plasma supernatant was retained. Methanol was added to the plasma for protein extraction. Quantitative analysis was performed on an Agilent 6460 triple quadrupole LC-MS/MS (tandem MS) using multiple reaction monitoring and electrospray ionization. For chromatographic separation, an Agilent Zorbax SB-C18 column (2.1 mm×50 mm; 5 μm) was used with a mobile phase gradient of 20–100% methanol in 0.1% formic acid and 5 mM ammonium formate at a flow rate of 0.4 ml/min. Plasma drug concentrations were quantified against a calibration curve of known drug concentrations ranging from 10 to 10000 nM, with quality controls included at 65, 650 and 6500 nM. To prevent contamination from previous samples, a methanol slug was run between each plasma sample. Pharmacokinetic parameters were determined by noncompartmental analysis using WinNonlin 5.3 software. |
| 参考文献 | |
| 其他信息 |
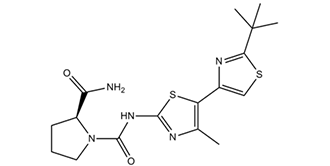
(2S)-N1-[5-(2-tert-butyl-4-thiazolyl)-4-methyl-2-thiazolyl]pyrrolidine-1,2-dicarboxamide is a proline derivative.
Genetic alterations in PI3K (phosphoinositide 3-kinase) signalling are common in cancer and include deletions in PTEN (phosphatase and tensin homologue deleted on chromosome 10), amplifications of PIK3CA and mutations in two distinct regions of the PIK3CA gene. This suggests drugs targeting PI3K, and p110α in particular, might be useful in treating cancers. Broad-spectrum inhibition of PI3K is effective in preventing growth factor signalling and tumour growth, but suitable inhibitors of p110α have not been available to study the effects of inhibiting this isoform alone. In the present study we characterize a novel small molecule, A66, showing the S-enantiomer to be a highly specific and selective p110α inhibitor. Using molecular modelling and biochemical studies, we explain the basis of this selectivity. Using a panel of isoform-selective inhibitors, we show that insulin signalling to Akt/PKB (protein kinase B) is attenuated by the additive effects of inhibiting p110α/p110β/p110δ in all cell lines tested. However, inhibition of p110α alone was sufficient to block insulin signalling to Akt/PKB in certain cell lines. The responsive cell lines all harboured H1047R mutations in PIK3CA and have high levels of p110α and class-Ia PI3K activity. This may explain the increased sensitivity of these cells to p110α inhibitors. We assessed the activation of Akt/PKB and tumour growth in xenograft models and found that tumours derived from two of the responsive cell lines were also responsive to A66 in vivo. These results show that inhibition of p110α alone has the potential to block growth factor signalling and reduce growth in a subset of tumours.[1] Cancer-specific mutations in the iSH2 (inter-SH2) and nSH2 (N-terminal SH2) domains of p85alpha, the regulatory subunit of phosphatidylinositide 3-kinase (PI3K), show gain of function. They induce oncogenic cellular transformation, stimulate cellular proliferation, and enhance PI3K signaling. Quantitative determinations of oncogenic activity reveal large differences between individual mutants of p85alpha. The mutant proteins are still able to bind to the catalytic subunits p110alpha and p110beta. Studies with isoform-specific inhibitors of p110 suggest that expression of p85 mutants in fibroblasts leads exclusively to an activation of p110alpha, and p110alpha is the sole mediator of p85 mutant-induced oncogenic transformation. The characteristics of the p85 mutants are in agreement with the hypothesis that the mutations weaken an inhibitory interaction between p85alpha and p110alpha while preserving the stabilizing interaction between p85alpha iSH2 and the adapter-binding domain of p110alpha. [2] In in vitro studies class-I PI3Ks (phosphoinositide 3-kinases), class-II PI3Ks and mTOR (mammalian target of rapamycin) have all been described as having roles in the regulation of glucose metabolism. The relative role each plays in the normal signalling processes regulating glucose metabolism in vivo is less clear. Knockout and knockin mouse models have provided some evidence that the class-I PI3K isoforms p110α, p110β, and to a lesser extent p110γ, are necessary for processes regulating glucose metabolism and appetite. However, in these models the PI3K activity is chronically reduced. Therefore we analysed the effects of acutely inhibiting PI3K isoforms alone, or PI3K and mTOR, on glucose metabolism and food intake. In the present study impairments in glucose tolerance, insulin tolerance and increased hepatic glucose output were observed in mice treated with the pan-PI3K/mTOR inhibitors PI-103 and NVP-BEZ235. The finding that ZSTK474 has similar effects indicates that these effects are due to inhibition of PI3K rather than mTOR. The p110α-selective inhibitors PIK75 and A66 also induced these phenotypes, but inhibitors of p110β, p110δ or p110γ induced only minor effects. These drugs caused no significant effects on BMR (basal metabolic rate), O2 consumption or water intake, but BEZ235, PI-103 and PIK75 did cause a small reduction in food consumption. Surprisingly, pan-PI3K inhibitors or p110α inhibitors caused reductions in animal movement, although the cause of this is not clear. Taken together these studies provide pharmacological evidence to support a pre-eminent role for the p110α isoform of PI3K in pathways acutely regulating glucose metabolism.[3] |
| 分子式 |
C17H23N5O2S2
|
|---|---|
| 分子量 |
393.5268
|
| 精确质量 |
393.129
|
| 元素分析 |
C, 51.89; H, 5.89; N, 17.80; O, 8.13; S, 16.29
|
| CAS号 |
1166227-08-2
|
| 相关CAS号 |
1166227-08-2
|
| PubChem CID |
42636535
|
| 外观&性状 |
Off-white to light yellow solid powder
|
| 密度 |
1.4±0.1 g/cm3
|
| 折射率 |
1.640
|
| LogP |
0.63
|
| tPSA |
162.17
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
26
|
| 分子复杂度/Complexity |
556
|
| 定义原子立体中心数目 |
1
|
| SMILES |
S1C([H])=C(C2=C(C([H])([H])[H])N=C(N([H])C(N3C([H])([H])C([H])([H])C([H])([H])[C@@]3([H])C(N([H])[H])=O)=O)S2)N=C1C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H]
|
| InChi Key |
HBPXWEPKNBHKAX-NSHDSACASA-N
|
| InChi Code |
InChI=1S/C17H23N5O2S2/c1-9-12(10-8-25-14(20-10)17(2,3)4)26-15(19-9)21-16(24)22-7-5-6-11(22)13(18)23/h8,11H,5-7H2,1-4H3,(H2,18,23)(H,19,21,24)/t11-/m0/s1
|
| 化学名 |
(2S)-1-N-[5-(2-tert-butyl-1,3-thiazol-4-yl)-4-methyl-1,3-thiazol-2-yl]pyrrolidine-1,2-dicarboxamide
|
| 别名 |
A-66; A66; 1166227-08-2; A66; (S)-N1-(2-(tert-butyl)-4'-methyl-[4,5'-bithiazol]-2'-yl)pyrrolidine-1,2-dicarboxamide; A 66; CHEMBL3218581; (2S)-1-N-[5-(2-tert-butyl-1,3-thiazol-4-yl)-4-methyl-1,3-thiazol-2-yl]pyrrolidine-1,2-dicarboxamide; 1,2-Pyrrolidinedicarboxamide, N1-[2-(1,1-dimethylethyl)-4'-methyl[4,5'-bithiazol]-2'-yl]-, (2S)-; A 66
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~79 mg/mL (~200.7 mM)
Water: ~1 mg/mL (~2.5 mM) Ethanol: ~4 mg/mL (~26.8 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.35 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (6.35 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (6.35 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 15% Captisol: 8mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5411 mL | 12.7055 mL | 25.4110 mL | |
| 5 mM | 0.5082 mL | 2.5411 mL | 5.0822 mL | |
| 10 mM | 0.2541 mL | 1.2706 mL | 2.5411 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
|
|
|