| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |
|
| 靶点 |
β1 adrenoceptor
β1-adrenoceptor (Ki = 0.3 nM) [2] β2-adrenoceptor (Ki = 30 nM) [2] |
|---|---|
| 体外研究 (In Vitro) |
醋丁洛尔抑制大鼠脑 P2 级分中的 NA 摄取,IC50 为 0.25 mM。 Acebutolol 对 125I 标记的 CYP 与人体脂肪细胞膜的结合产生浓度依赖性抑制,并且能够完全取代所有特异性结合的放射性配体。 Acebutolol 完全抑制 1 μM 异丙肾上腺素引发的脂肪分解活性。阿布洛尔是一种具有低脂溶性的心脏选择性拮抗剂。醋丁洛尔不与LDL结合,对J774巨噬细胞中胆固醇酯的细胞内积累的抑制作用比与LDL结合的阿普洛尔和奥普洛尔更强。
盐酸醋丁洛尔(Acebutolol HCl)是具有弱内在拟交感活性的选择性β1-肾上腺素受体拮抗剂。体外受体结合实验中,它对β1受体的选择性是β2受体的约100倍[2] 在表达β1受体的细胞中,它抑制异丙肾上腺素诱导的环磷酸腺苷(cAMP)积累,IC50为0.7 nM,而对表达β2受体的细胞影响极小(IC50 > 20 nM)[2] |
| 体内研究 (In Vivo) |
大鼠单次静脉注射醋丁洛尔(10 mg/kg)后,血浆清除率为 61.9 mL/min/kg,分布容积为 9.6 L/kg,消除半衰期为 1.8 小时。大鼠单次静脉注射醋丁洛尔(50 mg/kg)后,血浆清除率为 46.5 mL/min/kg,分布容积为 9.5 L/kg,消除半衰期为 2.3 小时。在 Sprague-Dawley 大鼠中,测量 1 分钟和 10 分钟后,醋丁洛尔 (30 mg/kg) 的心输出量分别降低 65% 和 31%。与 Sprague-Dawley 大鼠的基线值相比,醋丁洛尔 (30 mg/kg) 在 1 分钟或 10 分钟测量后显着降低了大多数器官的局部血流量 (RBF)。
在正常血压大鼠中,口服盐酸醋丁洛尔(Acebutolol HCl)(10、30 mg/kg/天,连续7天)剂量依赖降低静息心率(15-25%)和心输出量(10-18%)。30 mg/kg/天时,肾血流量减少约12%,但对脑或骨骼肌血流量无显著影响[3] 在慢性心力衰竭临床前模型中,盐酸醋丁洛尔(Acebutolol HCl)(20 mg/kg/天,口服,连续4周)通过降低左心室舒张末期容积和增加射血分数约20%改善心功能;同时通过抑制β1-肾上腺素受体介导的心肌肥大,减轻心肌重构[2] |
| 酶活实验 |
β1/β2-肾上腺素受体放射性配体结合实验:从豚鼠心脏(富含β1受体)和肺(富含β2受体)组织制备膜匀浆,将匀浆与[3H]-二氢阿普洛尔(非选择性β受体配体)及不同浓度的盐酸醋丁洛尔(Acebutolol HCl)(0.01-100 nM)在25°C孵育90分钟。通过玻璃纤维滤膜快速过滤分离结合态和游离态配体,用冰浴缓冲液洗涤滤膜后,通过闪烁计数器测定放射性强度,基于竞争结合曲线计算各受体亚型的Ki值[2]
cAMP积累实验:将表达人β1或β2肾上腺素受体的中国仓鼠卵巢(CHO)细胞接种到96孔板,用盐酸醋丁洛尔(Acebutolol HCl)(0.1-100 nM)处理30分钟,再用异丙肾上腺素(1 μM)刺激15分钟。裂解细胞后,采用竞争性酶免疫测定法检测cAMP水平,计算cAMP抑制的IC50值[2] |
| 细胞实验 |
大鼠心输出量与区域血流量研究:成年雄性正常血压大鼠随机分为对照组和处理组,盐酸醋丁洛尔(Acebutolol HCl)悬浮于0.5%羧甲基纤维素中,以10或30 mg/kg/天剂量口服给药,连续7天。第8天,大鼠麻醉后,采用热稀释法测量心输出量;通过放射性微球注射和组织放射性计数评估区域血流量(肾、脑、骨骼肌)[3]
大鼠药代动力学研究:成年雄性大鼠禁食过夜,单次口服给予盐酸醋丁洛尔(Acebutolol HCl)(20 mg/kg)的蒸馏水溶液。给药后0.25、0.5、1、2、4、6、8、12和24小时通过尾静脉采集血样,离心分离血浆,采用手性检测的高效液相色谱(HPLC)测定醋丁洛尔对映体浓度[1] |
| 动物实验 |
Dissolved in saline; 10 mg/kg; i.v. injection
Sprague–Dawley rats Rat cardiac output and regional blood flow study: Adult male normotensive rats are randomly divided into control and treatment groups. Acebutolol HCl is suspended in 0.5% carboxymethylcellulose and administered orally at 10 or 30 mg/kg/day for 7 days. On day 8, rats are anesthetized, and cardiac output is measured using a thermodilution technique. Regional blood flow (renal, cerebral, skeletal muscle) is assessed by radioactive microsphere injection and tissue radioactivity counting [3] Rat pharmacokinetic study: Adult male rats are fasted overnight and administered a single oral dose of Acebutolol HCl (20 mg/kg) as a solution in distilled water. Blood samples are collected via tail vein at 0.25, 0.5, 1, 2, 4, 6, 8, 12, and 24 hours post-administration. Plasma is separated by centrifugation, and concentrations of acebutolol enantiomers are determined by high-performance liquid chromatography (HPLC) with chiral detection [1] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Distribution of acebutolol hydrochloride into body tissue and fluids has not been fully characterized. Following IV administration in rats, acebutolol is distributed extensively into many tissues, including heart, liver, kidneys, lungs, intestines, stomach, and salivary glands, but only minimally into CSF or testes. Following oral administration of acebutolol hydrochloride in healthy individuals, acebutolol and, to a lesser extent, diacetolol, are distributed into saliva and minimally into CSF. Following oral administration of a single 300-mg dose of acebutolol hydrochloride, about 3-9% of the dose is distributed into bile within 24 hours, in approximately equivalent amounts as acebutolol and diacetolol. Peak biliary concentrations of acebutolol are approximately 60-100 times greater than peak plasma concentrations. Acebutolol and diacetolol readily cross the placenta and can accumulate in the fetus. In pregnant women receiving acebutolol, the mean acebutolol and diacetolol ratios of umbilical venous to maternal venous plasma concentrations were 0.8 (range: 0.5-1) and 0.6 (range: 0.3-0.8), respectively. Following IV administration, acebutolol is rapidly and widely distributed into the extravascular space and the apparent volume of distribution of the drug in healthy adults is approximately 1.6-3 l/kg (range: 1-3.8 l/kg). In healthy individuals, the volume of distribution in the central compartment and at steady state averages 0.16-0.22 and approximately 1.2 l/kg, respectively, following IV administration. The apparent volume distribution may be decreased in geriatric patients. In vitro, acebutolol and diacetolol are approximately 11-35 and 6-9% bound, respectively, to plasma proteins at plasma acebutolol concentrations of 20-9,000 ng/ml. Acebutolol is approximately 50% bound to erythrocytes. For more Absorption, Distribution and Excretion (Complete) data for ACEBUTOLOL HYDROCHLORIDE (16 total), please visit the HSDB record page. Metabolism / Metabolites Acebutolol is rapidly and extensively metabolized in the liver. Acebutolol undergoes extensive hydrolysis of the butyramide group to form the desbutyl primary amine, acetolol, which is almost completely converted via N-acetylation to diacetolol. The extent of metabolism of acebutolol to diacetolol appears to be independent of the genetic acetylator phenotype of the patient. Diacetolol is equipotent to acebutolol and has a similar pharmacologic profile. Biological Half-Life Following single or mutiple oral doses of acebutolol hydrochloride, the elimination half-life of diacetolol reportedly averages 21.5 hours (range: 11-49 hours) or 32 hours (range: 17-54 hours) in patients with creatinine clearances of 6-56 or less than 5 ml/minute, respectively. Following a single oral dose in healthy adults, the half-life of acebutolol in the initial distrubution phase (t1/2 alpha) is about 3 hours and the half-life in the terminal phase (t1/2 beta) has been reported to average 11 hours (range: 6-12 hours). The half-lives of the two identified metabolites, diacetolol and acetolol, average 7.5 (range: 7-11 hours) and 3 hours, respectively, following a single oral dose of the drug. The half-life of acebutolol tends to be slightly prolonged following multiple rather than single doses. Following multiple-dose oral administration of acebutolol hyrochloride in healthy individuals (400 mg twice daily for 56 days), the elimination half-life of acebutolol average 13 hours (range: 9-20 hours). The elimination half-lives of acebutolol and diacetolol may be slightly iincreased in geriatric patients. The plasma elimination half-lives of acebutolol and diacetolol in neonates born to women receiving the drug during pregnancy ranged from 6-14 and from 24-30 hours, respectively, in the first 24 hours after birth; the half-life of diacetolol decreased to 12-16 hours during the second day. Neonatal urinary excretion of the drug and diacetolol was maximal during the first 24 hours after birth. Oral absorption: Acebutolol HCl has oral bioavailability of ~40% in rats. Plasma concentration-time profiles of both enantiomers (R- and S-acebutolol) showed multiple peaking, with the second peak occurring 4-6 hours post-administration [1] Distribution: It has a volume of distribution (Vdss) of ~1.2 L/kg in rats. Both enantiomers distribute equally into most tissues, with no significant tissue accumulation [1] Metabolism: It is partially metabolized in the liver to an active metabolite (diacetolol). In rats, ~30% of the oral dose is metabolized to diacetolol within 24 hours [1][2] Excretion: The elimination half-life (t1/2) of R-acebutolol is ~3.5 hours and S-acebutolol is ~4.2 hours in rats. Approximately 50% of the dose is excreted in urine (20% as unchanged drug, 30% as metabolites) and 40% in feces [1] Plasma protein binding: Acebutolol HCl has a plasma protein binding rate of ~25-30% in rats and humans [1][2] |
| 毒性/毒理 (Toxicokinetics/TK) |
Interactions
When acebutolol and a catecholamine-depleting drug (eg, reserpine) are administered concomitantly, the effects of the drugs may be additive. When acebutolol is administered with diuretics or other hypotensive agents, the hypotensive effect may be increased. This effect usually is used to therapeutic advantage, but careful adjustment of dosage is necessary when these drugs are used concomitantly. No substantial pharmacokinetic interactions between acebutolol and hydrochlorothiazide or hydralazine have been observed. Acebutolol has decreased the hypoglycemic action of glyburide in type II diabetic patients, presumably by decreasing insulin secretion. Nonsteroidal anti-inflammatory agents have blunted the hypotensive effects of beta-adrenergic blocking agents. A delay in the onset of isoniazid induced convulsions was found in rats pretreated with the beta 2-adrenoceptor blocker, butoxamine, and the nonspecific beta-blocker, propranolol. In these animals the convulsive responses were inhibited in a dose dependent manner. These compounds were found to be effective even after the induction of convulsions. The beta-1-blocker, acebutolol was able to protect rats only when injected prior to the challenge. The anticonvulsant effect of acebutolol and propranolol but not that of butoxamine was found to be enhanced in animals pretreated with a gamma-aminobutyric acid elevating agent, aminooxyacetic acid. The findings indicate that the gamma-aminobutyric acid mediated anticonvulsant action of aminooxyacetic acid seems to be additive with that resulting from beta-1 but not beta-2-blockade. Drug interaction studies with tolbutamide and warfarin indicated no influence on the therapeutic effects of these compounds. Digoxin and hydrochlorothiazide plasma levels were not affected by concomitant Sectral /acebutolol hydrochloride/ administration. The kinetics of sectral were not significantly altered by concomitant administration of hydrochlorothiazide, hydralazine, sulfinpyrazone, or oral contraceptives. Non-Human Toxicity Values LD50 Rat (male) oral 3.2 g/kg LD50 Rat (female) oral 5.2 g/kg LD50 Rat (male) iv 115 mg/kg LD50 Rat (female) iv 120 mg/kg) In subchronic toxicity study (28 days) in rats at oral doses up to 100 mg/kg/day, no significant hepatotoxicity, nephrotoxicity, or hematological abnormalities were observed [2] It caused mild bradycardia and hypotension at high doses (≥50 mg/kg/day, po) in rats, which were reversible upon drug withdrawal [3] |
| 参考文献 |
|
| 其他信息 |
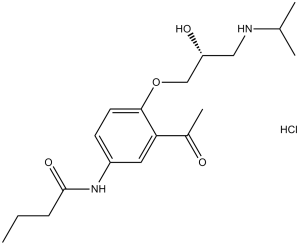
Acebutolol hydrochloride is the hydrochloride salt of acebutolol, prepared using equimolar amounts of acebutolol and hydrogen chloride. It has a role as an anti-arrhythmia drug, a beta-adrenergic antagonist, an antihypertensive agent and a sympathomimetic agent. It contains an acebutolol(1+).
Acebutolol Hydrochloride is the hydrochloride salt form of acebutolol, a synthetic butyranilide derivative with hypotensive and antiarrhythmic activity. Acebutolol acts as a cardioselective beta-adrenergic antagonist with little effect on bronchial receptors and has intrinsic sympathomimetic properties. Having stabilizing and quinidine-like effects on cardiac rhythm, Acebutolol is used in ventricular arrhythmias. Other indications include hypertension, alone or in combinations with other agents. (NCI04) A cardioselective beta-1 adrenergic antagonist with little effect on the bronchial receptors. The drug has stabilizing and quinidine-like effects on cardiac rhythm, as well as weak inherent sympathomimetic action. See also: Acebutolol (has active moiety). Mechanism of Action Acebutolol is a beta-selective adrenergic blocking agent and has pharmacologic actions similar to those of other beta-adrenergic blocking agents. At low dosages, acebutolol selectively inhibits response to adrenergic stimuli by competively blocking cardiac beta1-adrenergic receptors, while having little effect on the beta2-adrenergic receptors of bronchial and vascular smooth muscle. At high dosages (eg, greater than 80 mg daily), the selectively of acebutolol for beta-1-adrenergic receptors usually diminishes, and the drug will competively inhibit beta-1- and beta-2-adrenergic receptors. The beta1-selective blocking activity of acebutolol appears to be more pronounced in animals than in humans. In vivo studies in animals and humans indicate that the relative beta-1-adrenergic blocking activity of acebutolol, on a weight basis, is approximately 10-30% that of propranolol, as determined by inhibition of reflex tachycardia in animals or inhibition of exercise or tilt-induced or reflex tachycardia in healthy individuals. In addition to inhibiting access of physiologic or synthetic catecholamines to beta-adrenergic receptors, acebutolol exhibits mild intrinsic sympathomimetic activity (partial beta-agonist activity). Acebutolol also has a membrane-stabilizing effect on the heart, which is similar to that of quinidine but occurs only at high plasma concentrations and usually is not apparent at dosages used clinically. The pharmacologic effects of acebutolol results from both the unchanged drug and its major metabolite, diacetolol. Diacetolol is equipotent to acebutolol and, in animals, has greater beta-selective adrenergic blocking activity than the parent drug. Diacetolol also has weak intrinsic sympathomimetic activity but does not have substantial membrane-stabilizing activity. Diacetolol may contribute substantially to the observed effects of acebutolol, since plasma concentrations of the metabolite are consistently higher than those of the parent during acebutolol therapy. Therapeutic Uses Adrenergic beta-Antagonists; Anti-Arrhythmia Agents; Antihypertensive Agents; Sympatholytics Acebutolol ... /is/ indicated in the treatment of classic angina pectoris, also referred to as \"effort-associated angina\". /NOT included in US product labeling/ Acebutolol /is/ used in the treatment of mitral value prolapse syndrome. /NOT included in US product labeling/ Acebutolol ... /is/ used for thyrotoxicosis. /NOT included in US product labeling/ For more Therapeutic Uses (Complete) data for ACEBUTOLOL HYDROCHLORIDE (10 total), please visit the HSDB record page. Drug Warnings Abrupt withdrawal of acebutolol may exacerbate angina symptoms or precipitate myocardial infarction in patients with corornary artery disease. Therefore, patients receiving acebutolol (especially those with ischemic heart disease) should be warned not to interrupt or discontinue therapy without consulting their physician. When acebutolol therapy is discontinued, patients should be monitored carefully and advised to temporarily limit their physical activity. If exacerbation of angina occurs after acebutolol therapy is interrupted, antianginal therapy should be reinstituted promptly, and appropriate measures for the management of unstable angina pectoris should be initiated. Because coronary artery disease is common and may be unrecognized, it may be prudent not to discontinue acebutolol therapy abruptly, even in patients receiving the drug for conditions other than angina. Since beta-adrenergic blocking agents may reduce cardiac output and precipitate or aggravate the symptoms of arterial insufficiency in patients with peripheral or mesenteric vascular disease, acebutolol should be used with caution in these patients, and the patients should be observed for evidence of progression of arterial insufficiency. ... It is recommended that acebutolol be used with caution in patients with diabetes mellitus (especially those with labile diabetes) since the drug also may mask signs and symptoms of hypoglycemia (e.g., tachycardia, palpitation, blood pressure changes, tremor, feelings of anxiety, but not sweating) and may potentiate insulin-induced hypoglycemia. Since beta-adrenergic blocking agents may inhibit bronchodilation produced by endogenous catecholamines, the drugs generally should not be used in patients with bronchospastic disease; however, because of its relative beta 1-selective adrenergic blocking activity, acebutolol may be used with caution in patients with bronchospastic disease who do not respond to or cannot tolerate other hypotensive agents. For more Drug Warnings (Complete) data for ACEBUTOLOL HYDROCHLORIDE (22 total), please visit the HSDB record page. Acebutolol HCl is a cardioselective β1-adrenoceptor antagonist with weak intrinsic sympathomimetic activity [2] Its mechanism of action involves competitive blocking of cardiac β1-adrenoceptors, reducing heart rate, cardiac output, and myocardial oxygen demand, and inhibiting β1-mediated myocardial remodeling [2][3] Clinically indicated for the treatment of hypertension, ventricular arrhythmias, and chronic heart failure [2] The multiple peaking phenomenon in plasma concentration is attributed to enterohepatic circulation or variable absorption in the gastrointestinal tract [1] Its weak intrinsic sympathomimetic activity minimizes resting bradycardia, making it suitable for patients with mild bradycardia at baseline [2] |
| 分子式 |
C18H29CLN2O4
|
|
|---|---|---|
| 分子量 |
372.8869
|
|
| 精确质量 |
372.181
|
|
| 元素分析 |
C, 57.98; H, 7.84; Cl, 9.51; N, 7.51; O, 17.16
|
|
| CAS号 |
34381-68-5
|
|
| 相关CAS号 |
Acebutolol; 37517-30-9; Acebutolol-d7
|
|
| PubChem CID |
441307
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 沸点 |
564.1ºC at 760 mmHg
|
|
| 熔点 |
141-1430C
|
|
| LogP |
3.631
|
|
| tPSA |
87.66
|
|
| 氢键供体(HBD)数目 |
4
|
|
| 氢键受体(HBA)数目 |
5
|
|
| 可旋转键数目(RBC) |
10
|
|
| 重原子数目 |
25
|
|
| 分子复杂度/Complexity |
401
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
0
|
|
| InChi Key |
KTUFKADDDORSSI-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C18H28N2O4.ClH/c1-5-6-18(23)20-14-7-8-17(16(9-14)13(4)21)24-11-15(22)10-19-12(2)3;/h7-9,12,15,19,22H,5-6,10-11H2,1-4H3,(H,20,23);1H
|
|
| 化学名 |
N-[3-acetyl-4-[2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl]butanamide;hydrochloride
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.70 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (6.70 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (6.70 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: Saline: 30 mg/mL 配方 5 中的溶解度: 120 mg/mL (321.81 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6818 mL | 13.4088 mL | 26.8176 mL | |
| 5 mM | 0.5364 mL | 2.6818 mL | 5.3635 mL | |
| 10 mM | 0.2682 mL | 1.3409 mL | 2.6818 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT01514019 | Completed | Drug: Acebutolol Drug: Placebo |
Healthy | In-Jin Jang, MD, PhD | January 2012 | Phase 4 |
| NCT01743885 | Terminated | Drug: Acebutolol Drug: Propanolol |
Hemangioma | University Hospital, Montpellier | November 2012 | Phase 3 |