| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
The target of Aceclidine is the muscarinic acetylcholine receptor, particularly the M3 subtype.
|
|---|---|
| 体内研究 (In Vivo) |
In Vivo: Aceclidine被评估用于治疗眼屈光不正。在动物模型中,局部应用含Aceclidine的制剂可降低近视度数,通过屈光误差的变化来衡量。观察到该效果具有剂量依赖性,较高浓度的化合物能更显著地改善屈光状态 [1]
在半胆碱-3所致空间学习能力受损的实验中,给予Aceclidine可逆转该缺陷。这种逆转通过空间学习任务中的表现改善得以证实,表明其在增强与记忆和学习相关的胆碱能神经传递中发挥作用 [4] |
| 动物实验 |
Animal Protocol: For assessing effects on refractive errors, animals with induced myopia were treated with topical ophthalmic formulations containing Aceclidine. The formulations were applied to the eye(s) at specified frequencies (e.g., once or twice daily) for a defined period. Refractive error was measured using retinoscopy or similar techniques before and after treatment to evaluate efficacy [1]
In studies on spatial learning, animals were first administered hemicholinium-3 to impair spatial learning. Subsequently, Aceclidine was administered via a specified route (e.g., intraperitoneal injection) at a defined dose. Animals were then tested in spatial learning tasks (e.g., maze tests) to assess reversal of the impairment, with performance metrics recorded and compared to controls [4] |
| 毒性/毒理 (Toxicokinetics/TK) |
1979 rat LD50 subcutaneous 225 mg/kg Arzneimittel-Forschung. Drug Research., 18(320), 1968
1979 rat LD50 intravenous 45 mg/kg Arzneimittel-Forschung. Drug Research., 18(320), 1968 1979 mouse LD50 oral 165 mg/kg BEHAVIORAL: TREMOR; BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD; GASTROINTESTINAL: CHANGES IN STRUCTURE OR FUNCTION OF SALIVARY GLANDS Arzneimittel-Forschung. Drug Research., 18(320), 1968 1979 mouse LD50 intraperitoneal 78 mg/kg BEHAVIORAL: TREMOR; BEHAVIORAL: ANALGESIA Archives Internationales de Pharmacodynamie et de Therapie., 189(295), 1971 [PMID:5548767] 1979 mouse LD50 subcutaneous 102 mg/kg BEHAVIORAL: TREMOR; BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD; GASTROINTESTINAL: CHANGES IN STRUCTURE OR FUNCTION OF SALIVARY GLANDS Arzneimittel-Forschung. Drug Research., 18(320), 1968 |
| 参考文献 | |
| 其他信息 |
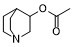
Acetic acid 1-azabicyclo[2.2.2]octan-3-yl ester is a member of quinuclidines.
Aceclidine has been marketed in Europe but has not been used clinically in the United States. It is used in the treatment of open-angle glaucoma and is a parasympathomimetic agent. Aceclidine is a cholinergic agent that acts as a modulator of muscarinic acetylcholine receptors, with a focus on the M3 subtype. It is formulated in storage-stable compositions, often with other excipients to enhance stability and efficacy, for topical ophthalmic use in treating refractive errors such as myopia |
| 分子式 |
C9H15NO2
|
|---|---|
| 分子量 |
169.22
|
| 精确质量 |
169.11
|
| 元素分析 |
C, 63.88; H, 8.93; N, 8.28; O, 18.91
|
| CAS号 |
827-61-2
|
| 相关CAS号 |
6109-70-2 (HCl racemic);827-61-2 (free base racemic);59653-40-6 (R-isomer);59653-42-8 (S-isomer);
|
| PubChem CID |
1979
|
| 外观&性状 |
Off-white to light yellow <34°C powder,>36°C liquid
|
| 沸点 |
221.5ºC at 760 mmHg
|
| 闪点 |
85ºC
|
| LogP |
0.581
|
| tPSA |
29.54
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
12
|
| 分子复杂度/Complexity |
185
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O=C(C)OC1C2CCN(CC2)C1
|
| InChi Key |
WRJPSSPFHGNBMG-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C9H15NO2/c1-7(11)12-9-6-10-4-2-8(9)3-5-10/h8-9H,2-6H2,1H3
|
| 化学名 |
1-Azabicyclo(2.2.2)octan-3-ol, acetate (ester)
|
| 别名 |
Glaucostat; Aceclidine; NSC-657843; aceclidine; 827-61-2; 3-Acetoxyquinuclidine; 3-Quinuclidinol acetate (ester); Aceclidina; Aceclidinum; 3-Quinuclidinol acetate; 1-Azabicyclo(2.2.2)octan-3-ol, acetate (ester); NSC657843; NSC 657843
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~590.95 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (14.77 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (14.77 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (14.77 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 5.9095 mL | 29.5473 mL | 59.0947 mL | |
| 5 mM | 1.1819 mL | 5.9095 mL | 11.8189 mL | |
| 10 mM | 0.5909 mL | 2.9547 mL | 5.9095 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05431543 | Completed | Drug: Aceclidine+Brimonidine combination ophthalmic solution |
Presbyopia Refractive Errors |
Alisyn Facemire | August 6, 2022 | Phase 2 |
| NCT05294328 | Completed | Drug: Aceclidine+Brimonidine combination ophthalmic solution |
Presbyopia Refractive Errors |
LENZ Therapeutics, Inc | May 5, 2022 | Phase 2 |
| NCT05936489 | Completed | Drug: Aceclidine + Brimonidine Drug: Aceclidine |
Eye Diseases Presbyopia |
LENZ Therapeutics, Inc | July 6, 2023 | Phase 1 |
| NCT03201562 | Completed Has Results | Drug: Aceclidine+tropicamide combination |
Presbyopia | LENZ Therapeutics, Inc | April 30, 2017 | Phase 2 |
|