| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 15mg |
|
||
| 100mg |
|
||
| 500mg |
|
||
| 5g |
|
||
| 10g |
|
||
| 25g |
|
||
| 50g |
|
||
| 100g |
|
||
| 200g |
|
||
| Other Sizes |
|

| 靶点 |
COX-2 (IC50 = 25.8 μM); COX-1 (IC50 =113.7 μM); cyclooxygenase-2
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
对乙酰氨基酚在体外抑制 COX-2 的选择性是 COX-1 的 4.4 倍(COX-1 的 IC50 为 113.7 μM,COX-2 的 IC50 为 25.8 μM)。口服药物治疗后的最大体外抑制率为 56% (COX-1) 和 83% (COX-2)。注射后至少 5 小时,对乙酰氨基酚血浆浓度保持高于 COX-2 的体外 IC50。对乙酰氨基酚的离体 IC50 值(COX-1:105.2 μM;COX-2:26.3 μM)与其体外 IC50 值相比效果良好。与其他理论不同,对乙酰氨基酚可抑制 COX-2 超过 80%,这意味着它的抑制程度与选择性 COX-2 抑制剂和非甾体类抗炎药物 (NSAID) 相似。不可能建立 >95% 的 COX-1 阻断,而 COX-1 阻断是抑制血小板功能所必需的[1]。根据 MTT 测定,50 mM 剂量的对乙酰氨基酚 (APAP) 显着 (p<0.001) 将细胞活力降低至 61.5±6.65%。有趣的是,当将对乙酰氨基酚/HV110 共同处理的细胞与对乙酰氨基酚处理的细胞进行比较时,细胞活力显着 (p<0.01) 增加至 79.7±2.47%[2]。
|
||
| 体内研究 (In Vivo) |
在动物模型中,对乙酰氨基酚可用于创建急性肝损伤的小鼠模型。
高剂量的对乙酰氨基酚(APAP)会导致急性肝损伤。在这项研究中,我们评估了柠檬醛在APAP诱导的小鼠肝毒性模型中的作用。测定肝功能标志物丙氨酸转氨酶(ALT)、天冬氨酸转氨酶(AST)、碱性磷酸酶(ALP)和γ-谷氨酰转移酶(γGT),以评估柠檬醛的保肝作用。肝脏用于测定髓过氧化物酶(MPO)活性和一氧化氮(NO)的产生,并用于组织学分析。在体外评估柠檬醛对白细胞迁移和抗氧化活性的影响。柠檬醛预处理显著降低了ALT、AST、ALP和γGT的水平、MPO活性和NO的产生。组织病理学分析显示,柠檬醛预处理后小鼠肝脏病变有所改善。柠檬醛抑制中性粒细胞迁移并显示抗氧化活性。我们的研究结果表明,柠檬醛可以保护肝脏免受APAP引起的肝毒性[3]。 |
||
| 酶活实验 |
三十多年来,对乙酰氨基酚(国际非专利药品名称,扑热息痛)一直被认为对外周前列腺素没有明显的抑制作用。同时,试图通过抑制中心环氧化酶(COX)-3来解释其作用的尝试被拒绝了。对乙酰氨基酚具有选择性COX-2抑制剂的功能,这一事实促使我们研究了它是否通过优先阻断COX-2起作用的假设。在5名接受单次1000mg口服剂量的志愿者中评估了对乙酰氨基酚的体外COX抑制和药代动力学。在人全血中离体和体外测量凝血诱导的血栓素B(2)和脂多糖诱导的前列腺素E(2),作为COX-1和COX-2活性的指标。在体外,对乙酰氨基酚对COX-2抑制的选择性是4.4倍(COX-1的IC(50)=113.7微摩尔/升;COX-2的IC(50)=25.8微摩尔/升)。口服该药物后,最大离体抑制率为56%(COX-1)和83%(COX-2)。给药后至少5小时,对乙酰氨基酚血浆浓度仍高于COX-2的体外IC(50)。对乙酰氨基酚的体外IC(50)值(COX-1:105.2微摩尔/L;COX-2:26.3微摩尔/L)与其体外IC(50中)值相比是有利的。与之前的概念相反,对乙酰氨基酚抑制COX-2的程度超过80%,即与非甾体抗炎药(NSAIDs)和选择性COX-2抑制剂相当。然而,没有实现与抑制血小板功能相关的>95%的COX-1阻断。我们的数据可以解释对乙酰氨基酚的镇痛和抗炎作用,以及与非甾体抗炎药相比其优越的整体胃肠道安全性。鉴于其对COX-2的显著抑制作用,最近定义的对乙酰氨基酚使用COX-2抑制剂的心血管警告也应考虑[1]。
|
||
| 细胞实验 |
本文以酪氨酸酶为癌症分子治疗靶点,研究了对乙酰氨基酚(APAP)对SK-MEL-28黑色素瘤细胞毒性的生化机制。我们的结果表明,在2小时的孵育下,APAP被酪氨酸酶代谢了87%。在酪氨酸酶氧化APAP的过程中,醌还原剂AA和NADH被显著消耗。APAP对SK-MEL-28、MeWo、SK-MEL-5、B16-F0和B16-F10黑色素瘤细胞的IC(50)(48小时)为100微M,而对BJ、Saos-2、SW-620和PC-3非黑色素瘤电池没有明显毒性,表明对黑色素瘤的选择性毒性。黄递酶抑制剂二香豆素和谷胱甘肽耗竭剂1-溴庚烷增强了APAP对SK-MEL-28细胞的毒性。AA和GSH能有效预防APAP诱导的黑色素瘤细胞毒性。三氟拉嗪和环孢素A是线粒体通透性转换孔的抑制剂,可显著预防APAP黑色素瘤细胞毒性。APAP导致SK-MEL-28细胞内GSH含量的时间和剂量依赖性下降,这先于细胞毒性。APAP导致SK-MEL-28细胞中ROS的形成,而双香豆素和1-溴庚烷会加剧这种形成,而胱孢菌素A和三氟拉嗪则会阻止这种形成。我们的研究表明,APAP是一种酪氨酸酶底物,细胞内GSH耗竭、ROS形成和诱导的线粒体毒性是APAP在SK-MEL-28细胞中的选择性毒性的原因[2]。
|
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption
Acetaminophen has 88% oral bioavailability and reaches its highest plasma concentration 90 minutes after ingestion. Peak blood levels of free acetaminophen are not reached until 3 hours after rectal administration of the suppository form of acetaminophen and the peak blood concentration is approximately 50% of the observed concentration after the ingestion of an equivalent oral dose (10-20 mcg/mL). The percentage of a systemically absorbed rectal dose of acetaminophen is inconsistent, demonstrated by major differences in the bioavailability of acetaminophen after a dose administered rectally. Higher rectal doses or an increased frequency of administration may be used to attain blood concentrations of acetaminophen similar to those attained after oral acetaminophen administration. Route of Elimination Acetaminophen metabolites are mainly excreted in the urine. Less than 5% is excreted in the urine as free (unconjugated) acetaminophen and at least 90% of the administered dose is excreted within 24 hours. Volume of Distribution Volume of distribution is about 0.9L/kg. 10 to 20% of the drug is bound to red blood cells. Acetaminophen appears to be widely distributed throughout most body tissues except in fat. Clearance Adults: 0.27 L/h/kg following a 15 mg/kg intravenous (IV) dose. Children: 0.34 L/h/kg following a 15 mg/kg intravenous (IV dose). Metabolism / Metabolites Acetaminophen is the major metabolite of _phenacetin_ and _acetanilid_. Acetaminophen is mainly metabolized in the liver by first-order kinetics and its metabolism of comprised of 3 pathways: conjugation with glucuronide, conjugation with sulfate, and oxidation through the cytochrome P450 enzyme pathway, mainly CYP2E1, to produce a reactive metabolite (N-acetyl-p-benzoquinone imine or NAPQI). At normal therapeutic doses, NAPQI undergoes fast conjugation with glutathione and is subsequently metabolized to produce both cysteine and mercapturic acid conjugates. High doses of acetaminophen (overdoses) can lead to hepatic necrosis due to the depletion of glutathione and of binding of high levels of reactive metabolite (NAPQI) to important parts of liver cells. The abovementioned damage to the liver can be prevented by the early administration of sulfhydryl compounds, for example, methionine and N-acetylcysteine. Biological Half-Life The half-life for adults is 2.5 h after an intravenous dose of 15 mg/kg. After an overdose, the half-life can range from 4 to 8 hours depending on the severity of injury to the liver, as it heavily metabolizes acetaminophen. The elimination half life is 1-3 hours after a therapeutic dose but may be greater than 12 hours after an overdose. |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Acetaminophen is an odorless compound with a slightly bitter taste. It is a common analgesic and antipyretic agent used for the relief of fever as well as aches and pains associated with many conditions. HUMAN EXPOSURE AND TOXICITY: Nausea, vomiting, and abdominal pain usually occur within 2-3 hours after ingestion of toxic doses of the drug. In severe poisoning, CNS stimulation, excitement, and delirium may occur initially. This may be followed by CNS depression, stupor, hypothermia, marked prostration, rapid shallow breathing, rapid weak irregular pulse, low blood pressure, and circulatory failure. When an individual has ingested a toxic dose of acetaminophen, the individual should be hospitalized for several days of observation, even if there are no apparent ill effects, because maximum liver damage and/or cardiotoxic effects usually do not become apparent until 2-4 days after ingestion of the drug. Other symptoms of acute poisoning include cerebral edema and nonspecific myocardial depression. Vascular collapse results from the relative hypoxia and from a central depressant action that occurs only with massive doses. Shock may develop if vasodilation is marked. Fatal seizures may occur. Coma usually precedes death, which may occur suddenly or may be delayed for several days. Biopsy of the liver reveals centralobular necrosis with sparing of the periportal area. There have been reports of acute myocardial necrosis and pericarditis in individuals with acetaminophen poisoning. Hypoglycemia, which can progress to coma have been reported in patients ingesting toxic doses of acetaminophen. Low prothrombin levels and thrombocytopenia have been reported in patients with acetaminophen poisoning. Skin reactions of an erythematous or urticarial nature which may be accompanied by fever and oral mucosal lesions also have been reported. For use anytime during pregnancy, 781 exposures were recorded, and possible associations with congenital dislocation of the hip (eight cases) and clubfoot (six cases) were found. There is inadequate evidence in humans for the carcinogenicity of acetaminophen. ANIMAL TOXICITY STUDIES: There is inadequate evidence in experimental animals for the carcinogenicity of acetaminophen. In rats fasted 24 hours and given a single dose of acetaminophen (2 g/kg) by gavage, liver necrosis around the central vein was noted at 9-12 hours and was much more extensive at 24 hours after treatment. In mice after dietary exposure to acetaminophen up to 6400 mg/kg daily for 13 weeks hepatotoxicity, organ weight changes and deaths were observed. Cats are particularly susceptible to acetaminophen intoxication, developing more diffuse liver changes, while hepatic centrilobular lesions found in dogs. High doses of acetaminophen caused testicular atrophy and delay in spermiogenesis in mice. Furthermore, reductions in the fertility and neonatal survival in mice were seen in the F0 generation and decreases in F1 pup weights were found at acetaminophen dose 1430 mg/kg. Acetaminophen was not mutagenic in Salmonella typhimurium assay with or without metabolic activation in six strains: TA1535, TA1537, TA1538, TA100, TA97 and TA98. In vitro and animal data indicate that small quantities of acetaminophen are metabolized by a cytochrome P-450 microsomal enzyme to a reactive intermediate metabolite (N-acetyl-p-benzoquinoneimine, N-acetylimidoquinone, NAPQI) which is further metabolized via conjugation with glutathione and ultimately excreted in urine as a mercapturic acid. It has been suggested that this intermediate metabolite is responsible for acetaminophen-induced liver necrosis in cases of overdose. Excipients found in liquid formulations of acetaminophen may decrease its liver toxicity. ECOTOXICITY STUDIES: Daphnia magna was the most susceptible among the test organisms to the environmental effects of acetaminophen. Acetaminophen has recently been identified as a promising snake toxicant to reduce brown tree snake populations on Guam, while posing only the minimal risks to non-target rodents, cats, pigs and birds. Hazardous Substances Data Bank (HSDB) Paracetamol toxicity is one of the most common causes of poisoning worldwide. The toxic effects of acetaminophen are due to a minor alkylating metabolite (N-acetyl-p-benzo-quinone imine – also known as NAPQI), not acetaminophen itself nor any of the other major metabolites. Cytochromes P450 2E1 and 3A4 convert approximately 5% of paracetamol to NAPQI. This toxic metabolite reacts with sulfhydryl groups on proteins and with glutathione (GSH). NAPQI depletes the liver's natural antioxidant glutathione and directly damages cells in the liver, leading to liver failure. In animal studies, hepatic glutathione must be depleted to less than 70% of normal levels before hepatotoxicity occurs. More specifically, oxidation by NAPQI of GSH to GSSG (oxidized glutathione) and the reduction of GSSG back to GSH by the NADPH-dependent glutathione reductase appear to be responsible for the rapid oxidation of NADPH that occurs in hepatocytes incubated with NAPQI. Risk factors for toxicity include excessive chronic alcohol intake, fasting or anorexia nervosa, and the use of certain drugs such as isoniazid. At usual doses, paracetamol is quickly detoxified by combining irreversibly with the sulfhydryl group of glutathione to produce a non-toxic conjugate that is eventually excreted by the kidneys. The toxic dose of paracetamol is highly variable. Hepatotoxicity Chronic therapy with acetaminophen in doses of 4 grams daily has been found to lead to transient elevations in serum aminotransferase levels in a proportion of subjects, generally starting after 3 to 7 days, and with peak values rising above 3-fold elevated in 39% of persons. These elevations are generally asymptomatic and resolve rapidly with stopping therapy or reducing the dosage, and in some instances resolve even with continuation at full dose (Case 1). While acetaminophen has few side effects when used in therapeutic doses, recent reports suggest that its standard use can result in severe hypersensitivity reactions including Stevens Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN). Both of these syndromes can be life-threatening and both may be accompanied by evidence of liver injury. However, the hepatic involvement is usually mild and marked only by asymptomatic mild-to-moderate elevations in serum aminotransferase levels. The best known form of hepatoxicity from acetaminophen is an acute, serious hepatocellular injury as a result of intentional or unintentional overdose. The injury is due to a direct, toxic effect of the high doses of acetaminophen. Acetaminophen hepatotoxicity most commonly arises after a suicide attempt using more than 7.5 grams (generally more than 15 grams) as a single overdose (Case 2). Hepatic injury generally starts 24 to 72 hours after the ingestion with marked elevations in serum ALT and AST (often to above 2000 U/L), followed at 48 to 96 hours by clinical symptoms: jaundice, confusion, hepatic failure and in some instances death. Evidence of renal insufficiency is also common. Serum aminotransferase levels fall promptly and recovery is rapid if the injury is not too severe. Similar injury can occur with high therapeutic or supratherapeutic doses of acetaminophen given over several days for treatment of pain and not as a purposeful suicidal overdose (Case 3). This form of acetaminophen hepatotoxicity is referred to as accidental or unintentional overdose, and usually occurs in patients who have been fasting, or are critically ill with a concurrent illness, alcoholism or malnutrition, or have preexisting chronic liver disease. Some cases of unintentional overdose occur in patients taking acetaminophen in combinations with controlled substances (oxycodone, codeine), who take more than recommended amounts over several days in attempts to control pain or withdrawal symptoms. Instances of unintentional overdose in children are often due to errors in calculating the correct dosage or use of adult sized tablets instead of child or infant formulations. Because acetaminophen is present in many products, both by prescription and over-the-counter, another problem occurs when a patient ingests full or high doses of several products unaware that several contain acetaminophen. Likelihood score: A[HD] (well established cause of liver injury, but severe cases occur only with high doses). Health Effects Skin rashes, blood disorders and a swollen pancreas have occasionally happened in people taking the drug on a regular basis for a long time. Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Acetaminophen is a good choice for analgesia, and fever reduction in nursing mothers. Giving acetaminophen and ibuprofen on a fixed schedule for 24 hours after vaginal delivery appears to increase the breastfeeding rate. Amounts in milk are much less than doses usually given to infants. Adverse effects in breastfed infants appear to be rare. ◉ Effects in Breastfed Infants A maculopapular rash on the upper trunk and face of a 2-month-old infant was probably caused by acetaminophen in breastmilk. The rash occurred after 2 days of therapy in the mother at a dose of 1 gram at bedtime. It subsided when the drug was discontinued and recurred 2 weeks later after another acetaminophen dose of 1 gram was taken by the mother. Two papers report 14 women who breastfed after taking acetaminophen or its prodrug phenacetin with no adverse effects to their infants. In a telephone follow-up study, mothers reported no side effects among 43 infants exposed to acetaminophen in breastmilk. Two clinicians speculated that breastmilk exposure to acetaminophen during breastfeeding might be a risk factor for asthma and wheezing in the breastfed infants based on their personal observations. However, these observations were uncontrolled and cannot be considered to be valid proof of an association. ◉ Effects on Lactation and Breastmilk A randomized study compared the use of ibuprofen 400 mg plus acetaminophen 1 gram every 6 hours for 24 hours to the same combination on demand after normal vaginal delivery. Women who received the analgesics on a fixed schedule were more likely to breastfeed their baby (98% vs 88%) than those receiving analgesics on demand, even though their average pain scores were not different. Toxicity Data LD50: 338 mg/kg (Oral, Mouse) (A308) LD50: 1944 mg/kg (Oral, Rat) (A308) In adults, single doses above 10 grams or 200 mg/kg of bodyweight, whichever is lower, have a reasonable likelihood of causing toxicity. A308: Wishart DS, Knox C, Guo AC, Cheng D, Shrivastava S, Tzur D, Gautam B, Hassanali M: DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2008 Jan;36(Database issue):D901-6. Epub 2007 Nov 29. PMID:18048412 Treatment In adults, the initial treatment for paracetamol overdose is gastrointestinal decontamination. Paracetamol absorption from the gastrointestinal tract is complete within two hours under normal circumstances, so decontamination is most helpful if performed within this timeframe. Gastric lavage, better known as stomach pumping, may be considered if the amount ingested is potentially life-threatening and the procedure can be performed within 60 minutes of ingestion. Acetylcysteine, when used early in the course of treatment, reduces morbidity and virtually eliminating mortality associated with even a massive acetaminophen overdose. (L1712) In patients who develop fulminant hepatic failure or who are otherwise expected to die from liver failure, the mainstay of management is liver transplantation. Protein Binding The binding of acetaminophen to plasma proteins is low (ranging from 10% to 25%), when given at therapeutic doses. |
||
| 参考文献 |
[1]. FASEB J.2008 Feb;22(2):383-90;
[2]. J Pharm Sci.2009 Apr;98(4):1409-25. [3]. Evid Based Complement Alternat Med. 2017;2017:1796209. |
||
| 其他信息 |
4-hydroxyacetanilide is an odorless white crystalline solid. Bitter taste. pH (saturated aqueous solution) about 6.
Paracetamol is a member of the class of phenols that is 4-aminophenol in which one of the hydrogens attached to the amino group has been replaced by an acetyl group. It has a role as a cyclooxygenase 2 inhibitor, a cyclooxygenase 1 inhibitor, a non-narcotic analgesic, an antipyretic, a non-steroidal anti-inflammatory drug, a cyclooxygenase 3 inhibitor, a xenobiotic, an environmental contaminant, a human blood serum metabolite, a hepatotoxic agent, a ferroptosis inducer and a geroprotector. It is a member of phenols and a member of acetamides. It is functionally related to a 4-aminophenol. Acetaminophen (paracetamol), also commonly known as Tylenol, is the most commonly taken analgesic worldwide and is recommended as first-line therapy in pain conditions by the World Health Organization (WHO). It is also used for its antipyretic effects, helping to reduce fever. This drug was initially approved by the U.S. FDA in 1951 and is available in a variety of forms including syrup form, regular tablets, effervescent tablets, injection, suppository, and other forms. Acetaminophen is often found combined with other drugs in more than 600 over the counter (OTC) allergy medications, cold medications, sleep medications, pain relievers, and other products. Confusion about dosing of this drug may be caused by the availability of different formulas, strengths, and dosage instructions for children of different ages. Due to the possibility of fatal overdose and liver failure associated with the incorrect use of acetaminophen, it is important to follow current and available national and manufacturer dosing guidelines while this drug is taken or prescribed. Acetaminophen is a widely used nonprescription analgesic and antipyretic medication for mild-to-moderate pain and fever. Harmless at low doses, acetaminophen has direct hepatotoxic potential when taken as an overdose and can cause acute liver injury and death from acute liver failure. Even in therapeutic doses, acetaminophen can cause transient serum aminotransferase elevations. View More
Acetaminophen is a natural product found in Streptomyces xiamenensis and Euglena gracilis with data available.
Theraflu is any of the commercial combination preparations, by Novartis, containing a combination of any of the following agents: the analgesic antipyretic acetaminophen, an antihistamine (chlorpheniramine maleate, diphenhydramine hydrochloride, doxylamine succinate or pheniramine maleate), the antitussive dextromethorphan maleate and/or a decongestant (phenylephrine hydrochloride or pseudoephedrine hydrochloride). Theraflu preparations are used to relieve symptoms of cold and flu. Acetaminophen exerts its actions by inhibiting prostaglandin synthesis. The antihistamines block the effects of histamine. Dextromethorphan exerts its activity by raising the threshold for coughing in the cough center. The decongestants are sympathomimetic agents that cause vasoconstriction mediated through alpha-adrenergic receptors. This reduces blood flow, decreases swelling and prevents nasal and sinus congestion. Acetaminophen, also known as paracetamol, is commonly used for its analgesic and antipyretic effects. Its therapeutic effects are similar to salicylates, but it lacks anti-inflammatory, antiplatelet, and gastric ulcerative effects. The excellent tolerability of therapeutic doses of paracetamol (acetaminophen) is a major factor in the very wide use of the drug. The major problem in the use of paracetamol is its hepatotoxicity after an overdose. Hepatotoxicity has also been reported after therapeutic doses, but critical analysis indicates that most patients with alleged toxicity from therapeutic doses have taken overdoses. Importantly, prospective studies indicate that therapeutic doses of paracetamol are an unlikely cause of hepatotoxicity in patients who ingest moderate to large amounts of alcohol. (A7820). Single doses of paracetamol are effective analgesics for acute postoperative pain and give rise to few adverse effects. (A7821). Acetaminophen (AAP) overdose and the resulting hepatotoxicity is an important clinical problem. In addition, AAP is widely used as a prototype hepatotoxin to study mechanisms of chemical-induced cell injury and to test the hepatoprotective potential of new drugs and herbal medicines. Because of its importance, the mechanisms of AAP-induced liver cell injury have been extensively investigated and controversially discussed for many years. Analgesic antipyretic derivative of acetanilide. It has weak anti-inflammatory properties and is used as a common analgesic, but may cause liver, blood cell, and kidney damage. Drug Indication In general, acetaminophen is used for the treatment of mild to moderate pain and reduction of fever. It is available over the counter in various forms, the most common being oral forms. Acetaminophen _injection_ is indicated for the management of mild to moderate pain, the management of moderate to severe pain with adjunctive opioid analgesics, and the reduction of fever. Because of its low risk of causing allergic reactions, this drug can be administered in patients who are intolerant to salicylates and those with allergic tendencies, including bronchial asthmatics. Specific dosing guidelines should be followed when administering acetaminophen to children. Drug Warnings The U.S. Food and Drug Administration (FDA) is informing the public that acetaminophen has been associated with a risk of rare but serious skin reactions. These skin reactions, known as Stevens-Johnson Syndrome (SJS), toxic epidermal necrolysis (TEN), and acute generalized exanthematous pustulosis (AGEP), can be fatal. Acetaminophen is a common active ingredient to treat pain and reduce fever; it is included in many prescription and over-the-counter (OTC) products. Reddening of the skin, rash, blisters, and detachment of the upper surface of the skin can occur with the use of drug products that contain acetaminophen. These reactions can occur with first-time use of acetaminophen or at any time while it is being taken. ... Anyone who develops a skin rash or reaction while using acetaminophen or any other pain reliever/fever reducer should stop the drug and seek medical attention right away. Anyone who has experienced a serious skin reaction with acetaminophen should not take the drug again and should contact their health care professional to discuss alternative pain relievers/fever reducers. Health care professionals should be aware of this rare risk and consider acetaminophen, along with other drugs already known to have such an association, when assessing patients with potentially drug-induced skin reactions. Reported Fatal Dose In adults, hepatic toxicity rarely has occurred with acute overdoses of less than 10 g, although hepatotoxicity has been reported in fasting patients ingesting 4-10 g of acetaminophen. Fatalities are rare with less than 15 g. Drug Tolerance Although psychologic dependence on acetaminophen may occur, tolerance and physical dependence do not appear to develop even with prolonged use. Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. 2181 Pharmacodynamics Animal and clinical studies have determined that acetaminophen has both antipyretic and analgesic effects. This drug has been shown to lack anti-inflammatory effects. As opposed to the _salicylate_ drug class, acetaminophen does not disrupt tubular secretion of uric acid and does not affect acid-base balance if taken at the recommended doses. Acetaminophen does not disrupt hemostasis and does not have inhibitory activities against platelet aggregation. Allergic reactions are rare occurrences following acetaminophen use. Mechanism of Action According to its FDA labeling, acetaminophen's exact mechanism of action has not been fully established - despite this, it is often categorized alongside NSAIDs (nonsteroidal anti-inflammatory drugs) due to its ability to inhibit the cyclooxygenase (COX) pathways. It is thought to exert central actions which ultimately lead to the alleviation of pain symptoms. One theory is that acetaminophen increases the pain threshold by inhibiting two isoforms of cyclooxygenase, COX-1 and COX-2, which are involved in prostaglandin (PG) synthesis. Prostaglandins are responsible for eliciting pain sensations. Acetaminophen does not inhibit cyclooxygenase in peripheral tissues and, therefore, has no peripheral anti-inflammatory effects. Though acetylsalicylic acid (aspirin) is an irreversible inhibitor of COX and directly blocks the active site of this enzyme, studies have shown that acetaminophen (paracetamol) blocks COX indirectly. Studies also suggest that acetaminophen selectively blocks a variant type of the COX enzyme that is unique from the known variants COX-1 and COX-2. This enzyme has been referred to as _COX-3_. The antipyretic actions of acetaminophen are likely attributed to direct action on heat-regulating centers in the brain, resulting in peripheral vasodilation, sweating, and loss of body heat. The exact mechanism of action of this drug is not fully understood at this time, but future research may contribute to deeper knowledge. Acetaminophen produces analgesia and antipyresis by a mechanism similar to that of salicylates. Unlike salicylates, however, acetaminophen does not have uricosuric activity. There is some evidence that acetaminophen has weak anti-inflammatory activity in some nonrheumatoid conditions (e.g., in patients who have had oral surgery). ... Acetaminophen lowers body temperature in patients with fever but rarely lowers normal body temperature. The drug acts on the hypothalamus to produce antipyresis; heat dissipation is increased as a result of vasodilation and increased peripheral blood flow. American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 2211 |
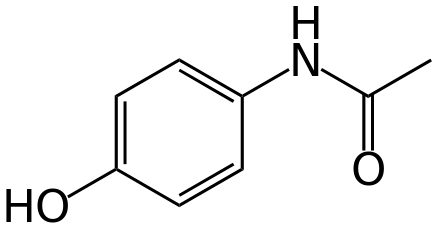
| 分子式 |
C8H9NO2
|
|
|---|---|---|
| 分子量 |
151.16
|
|
| 精确质量 |
151.063
|
|
| 元素分析 |
C, 63.56; H, 6.00; N, 9.27; O, 21.17
|
|
| CAS号 |
103-90-2
|
|
| 相关CAS号 |
Acetaminophen;103-90-2
|
|
| PubChem CID |
1983
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.3±0.1 g/cm3
|
|
| 沸点 |
387.8±25.0 °C at 760 mmHg
|
|
| 熔点 |
168-172 °C(lit.)
|
|
| 闪点 |
188.4±23.2 °C
|
|
| 蒸汽压 |
0.0±0.9 mmHg at 25°C
|
|
| 折射率 |
1.619
|
|
| LogP |
0.34
|
|
| tPSA |
49.33
|
|
| InChi Key |
RZVAJINKPMORJF-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C8H9NO2/c1-6(10)9-7-2-4-8(11)5-3-7/h2-5,11H,1H3,(H,9,10)
|
|
| 化学名 |
Acetamide, N-(4-hydroxyphenyl)-
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 该产品在溶液状态不稳定,请现配现用。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 6.67 mg/mL (44.13 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。
配方 2 中的溶解度: 10 mg/mL (66.16 mM) in 0.5% CMC-Na/saline water (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 View More
配方 3 中的溶解度: 10 mg/mL (66.16 mM) in saline 0.5% Tween-80 (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 6.6155 mL | 33.0775 mL | 66.1551 mL | |
| 5 mM | 1.3231 mL | 6.6155 mL | 13.2310 mL | |
| 10 mM | 0.6616 mL | 3.3078 mL | 6.6155 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02911961 | Withdrawn | Drug: Acetaminophen | Acetaminophen Exposure | Denver Health and Hospital Authority | August 2021 | Phase 4 |
| NCT05557344 | Recruiting | Drug: Acetaminophen IV Drug: Acetaminophen |
Pain | Dr. Niina Kleiber | April 21, 2021 | Phase 4 |
| NCT05246644 | Recruiting | Drug: acetaminophen | Delirium | McGill University Health Centre/Research Institute of the McGill University Health Centre |
June 6, 2023 | Phase 3 |
| NCT03020875 | Enrolling by invitation | Drug: Ofirmev Drug: Per Os Acetaminophen |
Multimodal Analgesic Approach | Hospital for Special Surgery, New York | January 2017 | Phase 4 |
|
|
|