| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
| 靶点 |
p38α (IC50 < 1 μM)
Acumapimod (BCT-197) targets p38α MAPK (IC50 = 0.13 μM) and p38β MAPK (IC50 = 0.17 μM); [1] Acumapimod (BCT-197) targets p38 MAPK [2] |
|---|---|
| 体外研究 (In Vitro) |
Acumapimod 是一种 p38α 抑制剂,IC50 值低于 1 μM。BCT197是一种口服低分子量p38抑制剂,目前正在开发中,用于治疗多种炎症性疾病,包括COPD。[2]
在无细胞激酶实验中,Acumapimod (BCT-197) 选择性抑制p38α和p38β亚型,IC50值分别为0.13 μM和0.17 μM,而对p38γ(IC50 > 10 μM)和p38δ(IC50 > 10 μM)的活性极低[1] - 用浓度范围为0.1 μM至10 μM的Acumapimod (BCT-197) 处理脂多糖(LPS)刺激的人外周血单核细胞(PBMC),可剂量依赖性减少促炎细胞因子的分泌,包括TNF-α(IC50 = 0.3 μM)、IL-6(IC50 = 0.5 μM)和IL-1β(IC50 = 0.4 μM)[1] - 用1 μM的Acumapimod (BCT-197) 孵育人支气管上皮细胞(HBEC),Western blot分析显示可抑制TNF-α诱导的p38 MAPK及其下游底物(如ATF-2)的磷酸化;同时,qPCR和ELISA结果显示基质金属蛋白酶-9(MMP-9)的mRNA和蛋白表达水平降低[1] |
| 体内研究 (In Vivo) |
Acumapimod (BCT-197)是一种口服低分子量 p38 抑制剂,正在开发用于口服治疗炎症性疾病,例如慢性阻塞性肺病 (COPD) 和其他炎症性疾病。 Acumapimod (BCT-197)(第 1 天和第 6 天 75 mg)在短时间内间歇给药,显示 COPD 患者的肺功能显着改善[2]。
当在体内检查Acumapimod (BCT-197),并与Vehicle治疗的动物进行比较时,观察到Acumapimod (BCT-197)浓度与慢性阻塞性肺疾病急性加重的临床试验中使用的浓度相关,体重减轻,生存改善和病毒控制不受损;当Acumapimod (BCT-197)浓度较高时,这些效应减弱。 结论:与Vehicle治疗相比,Acumapimod (BCT-197)(以临床相关浓度给药)改善了流感小鼠模型的预后。这是令人鼓舞的,因为使用先天炎症途径抑制剂可能会引起对感染调节的负面影响的担忧。https://pubmed.ncbi.nlm.nih.gov/29458547/ 在LPS诱导的小鼠急性肺损伤模型中,腹腔注射Acumapimod (BCT-197)(10 mg/kg,LPS攻击前30分钟给药)可显著减轻肺组织炎症:与溶媒对照组相比,支气管肺泡灌洗液(BALF)中中性粒细胞浸润减少约60%,巨噬细胞浸润减少约50%,且BALF中TNF-α(减少约70%)、IL-6(减少约65%)和MMP-9(减少约55%)的水平显著降低[1] - 在香烟烟雾诱导的大鼠慢性阻塞性肺疾病(COPD)模型中,口服给予Acumapimod (BCT-197)(3 mg/kg/天或10 mg/kg/天,连续4周)可剂量依赖性减轻气道重塑:高剂量处理组经组织病理学分析显示,支气管周围纤维化减少约40%,平滑肌肥厚减少约35%,黏液高分泌减少约50%;此外,高剂量组的肺功能参数(如0.1秒用力呼气量,FEV0.1)显著改善[1] |
| 酶活实验 |
BCT197抑制TNFα分泌,IC50为44µg/L。剂量前TNFα水平与IC50相关,并作为协变量建模(ΔOBJ 17)。在典型个体中,从基线开始的最大抑制并不完全,而是稳定在66%左右(Imax)。
在安慰剂治疗的受试者中,体外LPS诱导的tnf - α分泌的测量结果出现非平稳性(图2 c,插图)。不能排除脱落TNFα受体的昼夜节律周期性减弱对LPS的反应。因此,药物效应被描述为对振荡输入系统的抑制功能,其特征是物理频率为2π/24小时(Eq. 1),并且BCT197治疗对象和匹配安慰剂的所有可用tnf - α数据同时建模 p38 MAPK亚型特异性激酶实验:将重组人p38α、p38β、p38γ或p38δ激酶与特异性肽底物和ATP在反应缓冲液中混合。向反应体系中加入系列稀释的Acumapimod (BCT-197),于30°C孵育60分钟。使用发光检测系统测定肽底物的磷酸化水平,通过量效曲线的非线性回归分析计算IC50值[1] |
| 细胞实验 |
PBMC细胞因子分泌实验:从健康供体中分离人PBMC,以2×105个细胞/孔的密度接种到96孔板中。细胞用系列稀释的Acumapimod (BCT-197) 预孵育1小时,然后用LPS(1 μg/mL)刺激24小时。收集培养上清液,使用夹心ELISA试剂盒定量TNF-α、IL-6和IL-1β的浓度,绘制量效曲线以确定细胞因子抑制的IC50值[1]
- HBEC信号通路及MMP-9表达实验:将人支气管上皮细胞接种到6孔板中,培养至汇合。细胞饥饿培养16小时后,用1 μM的Acumapimod (BCT-197) 预孵育1小时,随后用TNF-α(10 ng/mL)刺激30分钟(用于信号通路分析)或24小时(用于MMP-9表达分析)。Western blot实验中,裂解细胞后通过SDS-PAGE分离蛋白,转移至膜上,用抗磷酸化p38 MAPK、总p38 MAPK、磷酸化ATF-2和GAPDH(内参)的抗体进行检测。qPCR实验中,提取总RNA并逆转录为cDNA,使用特异性引物定量MMP-9 mRNA水平,以GAPDH作为内参基因。上清液中的MMP-9蛋白通过ELISA测定[1] |
| 动物实验 |
Part 1 of study CBCT197A2101 was a randomized, double‐blind, placebo‐controlled, ascending single‐dose study to evaluate safety, tolerability, PK and PD of oral Acumapimod (BCT-197) in healthy subjects. Part 2 was a 14‐day, randomized, double‐blind, placebo‐controlled, ascending multiple dose study evaluating the PK and PD of oral Acumapimod (BCT-197). PD effect of BCT197 in Parts 1 and 2 was assessed by measuring TNFα levels in ex vivo LPS‐challenged blood samples. Details of the PK and PD sampling regimen, ex vivo LPS challenge, and bioanalysis of Acumapimod (BCT-197) and TNFα are provided in the Supplementary Methods. Part 3 of the study determined the effect of a single oral administration of BCT197 on serum TNFα levels after in vivo intravenous LPS challenge. Only PK data of this part were used, as it did not measure ex vivo LPS‐induced TNFα.
Subjects fasted for 10 hours prior to Acumapimod (BCT-197) administration and continued to fast for 4 hours postdosing. No fluid intake apart from the fluid given at the time of drug intake was allowed from 2 hours before until 2 hours after dosing. Drug administrations were oral solutions with doses ranging from 0.1 to 3 mg, and tablets at doses of 5 mg and higher.[2]
Mouse LPS-induced acute lung injury model: Male C57BL/6 mice (6-8 weeks old) were randomly divided into vehicle control, LPS alone, and Acumapimod (BCT-197) (10 mg/kg) groups. The drug was dissolved in 10% DMSO + 90% saline and administered via intraperitoneal injection 30 minutes before intranasal instillation of LPS (5 mg/kg). Mice were euthanized 24 hours after LPS challenge, and bronchoalveolar lavage fluid (BALF) was collected for cell counting and cytokine measurement; lung tissues were harvested for histopathological analysis [1] - Rat cigarette smoke-induced COPD model: Male Sprague-Dawley rats (12 weeks old) were exposed to cigarette smoke (10 cigarettes/day, 5 days/week) for 4 weeks to induce COPD. Concurrently, rats were treated with Acumapimod (BCT-197) at doses of 3 mg/kg/day or 10 mg/kg/day, or vehicle (0.5% methylcellulose), via oral gavage once daily. At the end of treatment, lung function was assessed using a whole-body plethysmograph, and rats were euthanized to collect lung tissues for histopathological analysis and measurement of inflammatory markers [1] |
| 药代性质 (ADME/PK) |
Drug administrations were oral solutions with doses up to 3 mg, and tablets at doses of 5 mg and higher. The population PK parameters along with their unexplained BSV and relative standard errors (RSE) are summarized in Table 1. Acumapimod (BCT-197) was found to be a low clearance drug (1.76 L/h), with linearity in oral drug clearance (CL/F) demonstrated over the entire dose range tested (0.1–75 mg). No relevant differences in relative bioavailability between these formulations were seen. Acumapimod (BCT-197) exhibited an apparent absorption plateau, with a tendency to less than dose‐proportional increase in peak drug concentration (Cmax). For tablets, the mixed‐order absorption model consisted of a first‐order process (Kt = 1.12 h−1), absorbing a fraction (fc) of on average 66% of dose, and a parallel zero‐order process characterized by a Rate of 2,300 µg/h (Table 1 ). A mixed‐order absorption model was significantly better than zero‐order (ΔOFV −509) or first‐order absorption (ΔOFV −403). Model parameters Rate and fc were found to be independent of dose. Addition of a random effect on Rate or fc did not improve the model fit. Oral absorption from the solution was parsimoniously described using a first‐order process (Ks). Limited data in the absorption phase impaired accurate estimation of Ks.[2]
Population predictions from linear disposition models showed overprediction in Cmax as well as underprediction in terminal disposition half‐life, particularly at low doses, despite dose linearity in CL/F. Conversely, the quasi‐equilibrium model with negligible receptor turnover from the peripheral compartment as depicted in Figure 2 captured well the apparent nonlinearity in tissue distribution (model equations in Supplementary Methods), and dropped the OFV by 123 points (Supplementary Table S2). The dissociation constant (Kd) and maximal binding (Bmax) were estimated to be respectively 367 µg and 500 µg. Limited‐capacity binding caused steady‐state volume of distribution (Vss/F) to increase with decreasing dose, with a limiting value of 132 L when the dose approached zero (Table 1). As expected, a competing quasi‐equilibrium model with nonlinear tissue binding into the central compartment showed bias in the structural model diagnostics (not shown).[2] Acumapimod (BCT-197) exhibited double peak behavior at about 18 to 24 hours postdose (Figure 1). This may indicate a late absorption window or drug redistribution by a shunt, possibly enterohepatic. The addition of a shunt feature further dropped OFV by 62 points. The full shunt model, however, was overparameterized (data too sparse). A reduction in degrees of freedom was achieved by fixing the duration of drug shunting (Tpump) as well as the rate of drug transfer from Ashunt into Aint (Db). Because model fit was insensitive to Kint1, it was set equal to Kt.[2] Oral bioavailability: In healthy human subjects, oral administration of Acumapimod (BCT-197) (single dose of 100 mg) resulted in an oral bioavailability of ~45% [2] - Plasma half-life (t1/2): In humans, the terminal plasma half-life of Acumapimod (BCT-197) was 12.3 ± 2.1 hours following a single oral dose of 100 mg [2] - Volume of distribution (Vd): The apparent volume of distribution of Acumapimod (BCT-197) in humans was 18.7 ± 3.2 L after a single oral dose of 100 mg [2] - Clearance (CL): Total plasma clearance of Acumapimod (BCT-197) in humans was 1.1 ± 0.2 L/h following a single oral dose of 100 mg [2] - Absorption: Peak plasma concentrations (Cmax = 2.8 ± 0.5 μg/mL) were achieved 2.5 ± 0.8 hours after oral administration of Acumapimod (BCT-197) (100 mg) in healthy subjects [2] |
| 毒性/毒理 (Toxicokinetics/TK) |
Continuous dosing of Acumapimod (BCT-197) 10 mg resulted in dose‐limiting acneiform skin rashes, whereas the drug was found to be well tolerated at single high doses up to 75 mg (data not shown). This observation, in addition to tolerance, raised the question whether Acumapimod (BCT-197) would be most efficacious in a continuous or intermittent regimen. Although the translational value of the ex vivo TNFα bioassay remains to be shown, efforts were made to assess the impact of dosing schedule on drug response through use of simulation.[2]
Plasma protein binding: Acumapimod (BCT-197) exhibited high plasma protein binding (92-94%) in human plasma, as determined by equilibrium dialysis [2] - Tolerability in humans: In a phase I clinical trial, single oral doses of Acumapimod (BCT-197) (up to 800 mg) and multiple doses (up to 400 mg/day for 14 days) were well-tolerated; the most common adverse events (AEs) were mild to moderate headache (15%), nausea (10%), and fatigue (8%), with no dose-limiting toxicity (DLT) observed [1][2] - No significant hepatotoxicity or nephrotoxicity was reported in clinical trials; serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine, and blood urea nitrogen (BUN) remained within normal ranges in all treated subjects [2] |
| 参考文献 | |
| 其他信息 |
Acumapimod is under investigation in clinical trial NCT02926326 (The Effect of Azithromycin on BCT197 Exposure in Healthy Male Volunteers).
Introduction: The JAK kinases are a family of four tyrosine receptor kinases that play a pivotal role in cytokine receptor signalling pathways via their interaction with signal transducers and activators of transcription proteins. Selective inhibitors of JAK kinases are viewed as of considerable potential as disease-modifying anti-inflammatory drugs for the treatment of rheumatoid arthritis. Areas covered: This article provides a review of the clinical development and available clinical results for those JAK inhibitors currently under investigation. Phase II data for four JAK inhibitors (baricitinib, decernotinib, filgotinib and INCB-039110) are contrasted with that reported for the recently approved JAK inhibitor tofacitinib. The preclinical data on these, in addition to peficitinib, ABT-494, INCB-047986 and AC-410 are also discussed, as are some of the inhibitors in preclinical development. Expert opinion: JAK inhibitors are effective in the treatment of rheumatoid arthritis as evidenced by several inhibitors enabling the majority of treated patients to achieve ACR20 responses, with baricitinib and INCB-039110 both effective when administered once daily. JAK inhibitors differ in isoform specificity profiles, with good efficacy achievable by selective inhibition of either JAK1 (filgotinib or INCB-039110) or JAK3 (decernotinib). It remains to be seen what selectivity provides the optimal side-effect profile and to what extent inhibition of JAK2 should be avoided. Keywords: JAK1 inhibitor; JAK3 inhibitor; baricitinib; decernotinib; filgotinib; peficitinib; rheumatoid arthritis; tofacitinib.[1] The p38 mitogen-activated protein kinase (p38) is a key signaling pathway involved in regulation of inflammatory cytokines. Unexpectedly, several clinical studies using p38 inhibitors found no convincing clinical efficacy in the treatment of chronic inflammation. It was the objective of this study to characterize the population pharmacokinetics (PK) of BCT197 in healthy volunteers and to examine the relationship between BCT197 exposure and pharmacodynamics (PD) measured as inhibition of ex vivo lipopolysaccharide (LPS)-induced tumor necrosis factor alpha (TNFα), a downstream marker of p38 activity. PK was characterized using a two-compartment model with mixed-order absorption and limited-capacity tissue binding. The PK-PD relationship revealed that suppression of TNFα was partly offset over time, despite continuous drug exposure. This may indicate a mechanism by which the inflammatory response acquires the ability to bypass p38. Simulations of posology dependence in drug effect suggest that an intermittent regimen may offer clinical benefit over continuous dosing and limit the impact of tolerance development.[2] Acumapimod (BCT-197) is a selective, orally active small-molecule inhibitor of p38α/β MAPK, developed for the treatment of chronic obstructive pulmonary disease (COPD) based on its anti-inflammatory and anti-remodeling properties [1] - The pharmacodynamic (PD) effect of Acumapimod (BCT-197) in humans is characterized by dose-dependent inhibition of LPS-induced TNF-α production in whole blood, with an EC50 of 0.8 μg/mL for TNF-α suppression [2] - A population pharmacokinetic-pharmacodynamic (PK-PD) model analysis of Acumapimod (BCT-197) in healthy subjects and COPD patients demonstrated that the drug’s anti-inflammatory effect (TNF-α inhibition) is correlated with plasma concentrations, and no significant tolerance to the PD effect was observed over 14 days of repeated dosing [2] - Acumapimod (BCT-197) has completed phase II clinical trials for COPD, showing potential benefits in reducing exacerbation rates and improving lung function in moderate-to-severe COPD patients, although further clinical development is ongoing [1] |
| 分子式 |
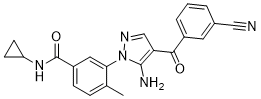
C22H19N5O2
|
|
|---|---|---|
| 分子量 |
385.42
|
|
| 精确质量 |
385.153
|
|
| 元素分析 |
C, 68.56; H, 4.97; N, 18.17; O, 8.30
|
|
| CAS号 |
836683-15-9
|
|
| 相关CAS号 |
|
|
| PubChem CID |
11338127
|
|
| 外观&性状 |
Yellow to orange solid powder
|
|
| 密度 |
1.4±0.1 g/cm3
|
|
| 沸点 |
675.6±55.0 °C at 760 mmHg
|
|
| 闪点 |
362.4±31.5 °C
|
|
| 蒸汽压 |
0.0±2.1 mmHg at 25°C
|
|
| 折射率 |
1.708
|
|
| LogP |
2.57
|
|
| tPSA |
114
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
5
|
|
| 可旋转键数目(RBC) |
5
|
|
| 重原子数目 |
29
|
|
| 分子复杂度/Complexity |
684
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
N#CC1C=C(C(C2=C(N)N(C3C(C)=CC=C(C(NC4CC4)=O)C=3)N=C2)=O)C=CC=1
|
|
| InChi Key |
VGUSQKZDZHAAEE-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C22H19N5O2/c1-13-5-6-16(22(29)26-17-7-8-17)10-19(13)27-21(24)18(12-25-27)20(28)15-4-2-3-14(9-15)11-23/h2-6,9-10,12,17H,7-8,24H2,1H3,(H,26,29)
|
|
| 化学名 |
3-[5-amino-4-(3-cyanobenzoyl)pyrazol-1-yl]-N-cyclopropyl-4-methylbenzamide
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.49 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (6.49 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (6.49 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5946 mL | 12.9729 mL | 25.9457 mL | |
| 5 mM | 0.5189 mL | 2.5946 mL | 5.1891 mL | |
| 10 mM | 0.2595 mL | 1.2973 mL | 2.5946 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
|