| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 靶点 |
DHODH (Dihydroorotate Dehydrogenase) (IC50: 1.2 nM for human DHODH enzyme activity; IC50: 3.7 nM for mouse DHODH enzyme activity) [1]
|
|---|---|
| 体外研究 (In Vitro) |
在源自实体瘤和血液肿瘤的肿瘤系中,AG-636 表现出终末生长抑制活性 [1]。
抑制DHODH酶活性 AG-636(0.1–10 nM)以剂量依赖方式抑制重组人及小鼠DHODH。在人DHODH IC50(1.2 nM)和小鼠DHODH IC50(3.7 nM)浓度下,酶活性降低50%;10 nM浓度下,抑制率分别达92%(人)和88%(小鼠),通过检测二氢乳清酸氧化的发光实验测定[1] - 血液系统恶性肿瘤细胞抗增殖活性 AG-636对多种血液肿瘤细胞系具有强效抗增殖作用。72小时MTT法检测IC50值为:Raji伯基特淋巴瘤8 nM、OCI-Ly10弥漫大B细胞淋巴瘤12 nM、K562慢性髓系白血病15 nM、HL-60急性髓系白血病18 nM、MM.1S多发性骨髓瘤22 nM。正常人外周血单个核细胞(PBMCs)敏感性极低(IC50 > 500 nM,100 nM浓度下细胞活力>80%)[1] - 抑制嘧啶从头合成 Raji细胞经AG-636(10 nM)处理24小时后,LC-MS/MS定量显示细胞内尿苷一磷酸(UMP)水平降低78%,胞苷三磷酸(CTP)水平降低65%,证实其抑制嘧啶从头合成(DHODH是该通路关键限速酶)[1] - 诱导凋亡与细胞周期阻滞 AG-636(10–30 nM)诱导Raji细胞凋亡:30 nM处理48小时后,Annexin V阳性细胞比例达62%(流式细胞术)。同时引起G1期细胞周期阻滞(20 nM浓度下G1期细胞从42%增至67%),S期比例从38%降至15%(碘化丙啶染色检测)[1] - 与其他抗癌药物的协同作用 Raji细胞经AG-636(5 nM)与阿糖胞苷(0.5 μM)联合处理后,细胞活力降低83%,而阿糖胞苷单独处理组降低35%,AG-636单独处理组降低42%。与维奈克拉(BCL-2抑制剂)和硼替佐米(蛋白酶体抑制剂)联合使用时也观察到协同效应[1] |
| 体内研究 (In Vivo) |
OCILY19 DLBCL 肿瘤异种移植模型对 AG-636(10-100 mg/kg;强饲法;每天两次;持续 14 天)显示出显着的肿瘤生长抑制作用 [1]。
血液系统恶性肿瘤异种移植模型的抗肿瘤疗效 荷Raji伯基特淋巴瘤异种移植瘤裸鼠,每日口服AG-636(10、25、50 mg/kg)连续21天,肿瘤生长抑制率分别为45%(10 mg/kg)、68%(25 mg/kg)和82%(50 mg/kg)。50 mg/kg组中位生存期延长14天(对照组为28天)[1] - OCI-Ly10 DLBCL异种移植模型的疗效 荷OCI-Ly10肿瘤的C.B-17 SCID小鼠,每日口服AG-636(25 mg/kg)连续21天,肿瘤生长抑制率71%,肿瘤重量减少65%。肿瘤组织分析显示UMP/CTP水平分别降低62%和58%,切割型caspase-3表达较对照组增加2.8倍[1] - 体内药效学验证 经AG-636(25 mg/kg)处理的Raji异种移植瘤小鼠,外周血和肿瘤组织中嘧啶核苷酸呈剂量依赖性降低。肿瘤浸润免疫细胞(CD8+ T细胞、NK细胞)未受显著影响,表明其对肿瘤免疫无明显干扰[1] |
| 酶活实验 |
DHODH酶活性实验
重组人/小鼠DHODH蛋白与AG-636(0.01–100 nM)在含二氢乳清酸(底物)和辅因子的反应缓冲液中孵育,37°C反应60分钟后,通过发光实验检测二氢乳清酸氧化产物,发光信号强度与酶活性成正比。根据量效曲线计算IC50值[1] - DHODH选择性实验 检测AG-636(1 μM)对50种代谢酶(包括其他嘧啶/嘌呤合成酶、激酶、磷酸酶)的抑制活性,其对DHODH的选择性>1000倍,对其他酶无显著抑制(<10%)[1] - 结合亲和力实验(ITC) 采用等温滴定量热法(ITC)测定AG-636与DHODH的结合亲和力。重组DHODH透析至实验缓冲液后,在25°C下将AG-636滴定至DHODH溶液中,检测结合过程中释放的热量,数据分析得出人DHODH的解离常数(KD)为0.8 nM[1] |
| 细胞实验 |
癌细胞抗增殖实验
血液肿瘤细胞系(Raji、OCI-Ly10、K562、HL-60、MM.1S)及正常PBMCs接种于96孔板(5×10³细胞/孔),过夜培养后加入0.1–1000 nM浓度的AG-636,孵育72小时。加入MTT试剂反应4小时后,检测570 nm波长吸光度,计算细胞活力和IC50值[1] - 嘧啶核苷酸定量实验 Raji细胞接种于6孔板(2×10⁶细胞/孔),经AG-636(1–30 nM)处理24小时后收集细胞并裂解,提取细胞内核苷酸(UMP、CTP),通过LC-MS/MS定量,峰面积按蛋白浓度归一化[1] - 凋亡与细胞周期实验 Raji细胞经AG-636(10–30 nM)处理48小时后,Annexin V-FITC/PI染色流式细胞术检测凋亡;细胞固定后经碘化丙啶染色,流式细胞术分析细胞周期分布[1] - Western blot分析 Raji细胞经AG-636(10–30 nM)处理24–48小时后裂解,蛋白经SDS-PAGE分离,转膜后用抗切割型caspase-3、切割型PARP、p21、周期蛋白D1及β-肌动蛋白(内参)抗体孵育,蛋白条带强度通过光密度法定量[1] |
| 动物实验 |
Animal/Disease Models: Transgenic female 6-8 oneweeks old CB17/Icr-Prkdcscid/IcrIcoCrl (CB17 SCID) mice [1] injected with OCILY19 cells [1]
Doses: 10 mg/kg, 30 mg/kg or 100 mg/kg Route of Administration: po (oral gavage); twice (two times) daily; for 14 days Experimental Results: Produced strong tumor growth inhibition in xenograft lymphoma model. Raji Burkitt lymphoma xenograft model Athymic nude mice (6–8 weeks old, 18–22 g) were acclimated for 7 days. Raji cells (5×10⁶ cells/mouse) were intravenously injected via the tail vein to establish systemic lymphoma. Three days post-inoculation, mice were randomized into groups (n=8/group). AG-636 was suspended in 0.5% carboxymethylcellulose sodium (CMC-Na) + 0.1% Tween 80 and administered by oral gavage at 10, 25, 50 mg/kg once daily for 21 days. Vehicle group received the same formulation without drug. Tumor burden was monitored via bioluminescence imaging (if luciferase-labeled cells were used) and survival was recorded daily. At study end, peripheral blood and tumor tissues were collected for nucleotide analysis and immunohistochemistry [1] - OCI-Ly10 DLBCL xenograft model C.B-17 SCID mice (6–8 weeks old) were subcutaneously injected with OCI-Ly10 cells (2×10⁷ cells/mouse) into the right flank. When tumors reached 100–150 mm³, mice were randomized (n=6/group) and treated with AG-636 (25 mg/kg, oral gavage) daily for 21 days. Tumor volume was measured every 2 days with calipers, and body weight was recorded weekly. At study end, tumors were excised, weighed, and processed for nucleotide quantification and western blot analysis [1] - Pharmacokinetic/toxicokinetic study Sprague-Dawley rats (200–250 g) were administered AG-636 via oral gavage (25 mg/kg) or intravenous injection (5 mg/kg). Blood samples were collected at 0.25, 0.5, 1, 2, 4, 8, 12, 24 hours post-dosing. Plasma was separated, and drug concentrations were measured by LC-MS/MS to calculate PK parameters (Cmax, t1/2, AUC, bioavailability) [1] |
| 药代性质 (ADME/PK) |
Oral bioavailability:58% in rats (25 mg/kg oral dose); 62% in mice (25 mg/kg oral dose) [1]
- Plasma half-life (t1/2):6.8 hours in rats (oral); 5.9 hours in mice (oral) [1] - Peak plasma concentration (Cmax):4.2 μM at 1 hour post-oral administration (25 mg/kg in mice); 3.8 μM at 1.5 hours (25 mg/kg in rats) [1] - Plasma protein binding rate:94.3% (in vitro human plasma); 93.1% (rat plasma) [1] - Tissue distribution:Highest concentrations in liver (7.6 μM), spleen (6.2 μM), and bone marrow (5.8 μM) at 2 hours post-oral dose (25 mg/kg in mice); minimal distribution in brain (0.3 μM) [1] - Metabolism and excretion:Metabolized primarily via CYP3A4 in the liver; 72% excreted in feces (parent drug + metabolites), 21% in urine within 72 hours [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
Acute toxicity:No mortality in mice after single oral dose up to 300 mg/kg; no obvious toxic signs (weight loss, lethargy, diarrhea) [1]
- Chronic toxicity:In 28-day repeat-dose study (mice: 10, 25, 50 mg/kg oral daily), no significant changes in body weight, hematological parameters (WBC, RBC, platelets, hemoglobin), or liver/kidney function markers (ALT, AST, BUN, creatinine) were observed. Histological examination of liver, kidney, bone marrow, spleen, and gastrointestinal tract showed no drug-related lesions [1] - Hematotoxicity:No significant suppression of bone marrow hematopoietic function at therapeutic doses (25 mg/kg); peripheral blood cell counts (neutrophils, lymphocytes, platelets) remained within normal range [1] - Drug-drug interaction potential:Weak inhibitor of CYP3A4 (IC50 > 10 μM in vitro); no induction of major CYP450 isoforms (CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP3A4) [1] |
| 参考文献 | |
| 其他信息 |
Mechanism of action:AG-636 is a selective, reversible inhibitor of DHODH, a key enzyme in the de novo pyrimidine synthesis pathway. By inhibiting DHODH, it blocks the conversion of dihydroorotate to orotate, reducing intracellular pyrimidine nucleotide (UMP, CTP, TTP) levels. Hematologic malignancies, which are highly dependent on de novo pyrimidine synthesis for rapid proliferation, undergo growth arrest and apoptosis due to nucleotide depletion [1]
- Therapeutic potential:Indicated for the treatment of hematologic malignancies, including Burkitt lymphoma, diffuse large B-cell lymphoma (DLBCL), chronic myeloid leukemia (CML), acute myeloid leukemia (AML), and multiple myeloma. It may also be used in combination with cytarabine, venetoclax, or bortezomib for enhanced efficacy [1] - Clinical-stage advantage:As a clinical-stage inhibitor, AG-636 exhibits favorable pharmacokinetics (oral bioavailability, long half-life) and safety profile, supporting its advancement into clinical trials for relapsed/refractory hematologic malignancies [1] - Selectivity feature:Highly selective for DHODH over other metabolic enzymes and kinases, minimizing off-target effects and improving tolerability compared to non-selective pyrimidine synthesis inhibitors [1] |
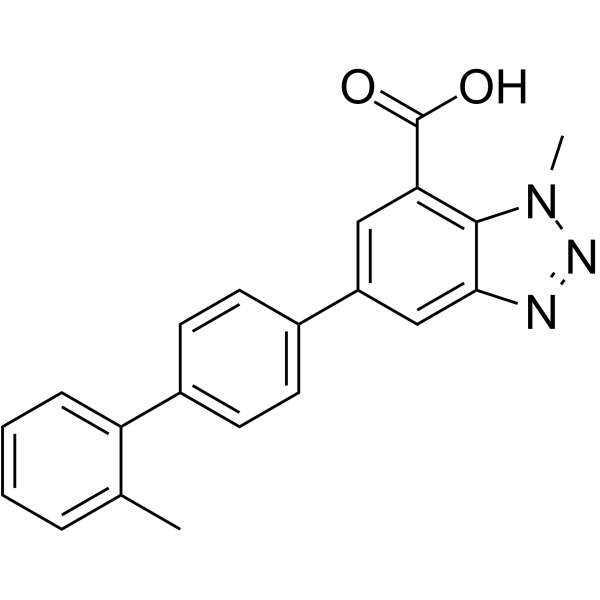
| 分子式 |
C21H17N3O2
|
|---|---|
| 分子量 |
343.386
|
| 精确质量 |
343.132
|
| 元素分析 |
C, 73.45; H, 4.99; N, 12.24; O, 9.32
|
| CAS号 |
1623416-31-8
|
| PubChem CID |
77461001
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.27±0.1 g/cm3
|
| 沸点 |
619.3±43.0 °C
|
| LogP |
4.3
|
| tPSA |
68
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
26
|
| 分子复杂度/Complexity |
506
|
| 定义原子立体中心数目 |
0
|
| SMILES |
OC(C1=CC(=CC2=C1N(C)N=N2)C1C=CC(=CC=1)C1C=CC=CC=1C)=O
|
| InChi Key |
GSBZRCGZLMBSNY-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C21H17N3O2/c1-13-5-3-4-6-17(13)15-9-7-14(8-10-15)16-11-18(21(25)26)20-19(12-16)22-23-24(20)2/h3-12H,1-2H3,(H,25,26)
|
| 化学名 |
1-methyl-5-(2'-methyl-[1,1'-biphenyl]-4-yl)-1H-benzo[d][1,2,3]triazole-7-carboxylic acid
|
| 别名 |
AG 636 AG-636 AG636
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~31.25 mg/mL (~91.01 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 2.08 mg/mL (6.06 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (6.06 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.9121 mL | 14.5607 mL | 29.1214 mL | |
| 5 mM | 0.5824 mL | 2.9121 mL | 5.8243 mL | |
| 10 mM | 0.2912 mL | 1.4561 mL | 2.9121 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。