| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 500mg |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
在 HFF 和 HUVEC 细胞中,别嘌呤醇(0、10、100 和 1000 µg/ml;17 小时)可以降低 HIF-1α 和 HIF-2α 蛋白的表达 [5]。在 24 小时内,别嘌呤醇(0、10、100 或 1000 µg/ml)会减弱 HUVEC 细胞的血管生成特征 [5]。
|
|---|---|
| 体内研究 (In Vivo) |
在小鼠中,别嘌呤醇(39 mg/kg;口服;每天一次,持续 21 天)显示出抗抑郁作用 [3]。在小鼠中,别嘌呤醇(10-400 mg/kg;腹腔注射)可引起抗伤害作用 [4]。
|
| 细胞实验 |
蛋白质印迹分析[5]
细胞类型: HFF、HUVEC 细胞 测试浓度: 0、10、100、1000 µg/ml 孵育持续时间:17小时 实验结果:HIF-1α和HIF-2α蛋白表达以剂量依赖性方式减弱。 |
| 动物实验 |
Animal/Disease Models: 20-30 g, male Swiss albino mouse [3]
Doses: 39 mg/kg Route of Administration: oral; one time/day for 21 days Experimental Results: diminished immobility time in FST, immobility time was 129.8± 10.5 seconds. Animal/Disease Models: 30-40 g, male adult Swiss albino mouse [4] Doses: 10, 50, 100, 200, 400 mg/kg Route of Administration: intraperitoneal (ip) injection Experimental Results: Dose dependence in tail flick and thermal stimulation Sexual antinociceptive effects plate. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
This drug is about 90% absorbed from the gastrointestinal tract. Peak plasma levels normally occur at 1.5 hours and 4.5 hours post-dose for allopurinol and oxipurinol respectively. Following one oral dose of 300 mg of allopurinol, maximum plasma levels of about 3 mcg/mL of allopurinol and 6.5 mcg/mL of oxipurinol were measured. Approximately 80% of orally ingested allopurinol is found excreted in the urine as various metabolites. About 20% of ingested allopurinol is excreted in the feces. Allopurinol and oxypurinol are both substrates for the enzyme xanthine oxidase, which is present in the cytoplasm of endothelial cells of capillaries, including sinusoids, with the highest activity demonstrated in the liver and intestinal lining. Tissue concentrations of allopurinol have not yet been reported in humans, however, it is probable that allopurinol and the metabolite oxypurinol would be measured in the highest concentrations in the abovementioned tissues. In animals, allopurinol concentrations are found to reach the highest levels in the blood, liver, intestine and heart, and lowest in the brain and lung tissues. Since allopurinol and its metabolites are mainly eliminated by the kidney, accumulation of this drug can occur in patients with renal dysfunction or failure, and the dose of allopurinol should, therefore, be reduced. With a creatinine clearance of 10 to 20 mL/min, a daily dosage of 200 mg of allopurinol is suitable. When the creatinine clearance is less than 10 mL/min, the daily dosage should not be higher than 100 mg. With severe renal impairment (creatinine clearance measured at less than 3 mL/min) a longer interval between doses may be required. Following oral administration, approximately 80-90% of a dose of allopurinol is absorbed from the GI tract. Peak plasma concentrations of allopurinol are reached 2-6 hours after a usual dose. Allopurinol is absorbed poorly following rectal administration of the drug as suppositories (in a cocoa butter or polyethylene glycol base). Plasma allopurinol or oxipurinol concentrations have been minimal or undetectable following such rectal administration. Following oral administration of single 100- or 300-mg dose of allopurinol in healthy adult males in one study, peak plasma allopurinol concentrations of about 0.5 or 1.4 ug/mL, respectively, occurred in about 1-2 hours, while peak oxypurinol (the active metabolite of allopurinol) concentrations of about 2.4 and 6.4 ug/mL, respectively, were reached within about 3-4 hours. In the same study, following iv infusion over 30 minutes of a single 100- or 300-mg dose of allopurinol (as allopurinol sodium), peak plasma concentrations of about 1.6 and 5.1 ug/mL, respectively, occurred in about 30 minutes, while peak oxypurinol concentrations of about 2.2 and 6.2 ug/mL, respectively, were reached within about 4 hours. Following intravenous administration in six healthy male and female subjects, allopurinol was rapidly eliminated from the systemic circulation primarily via oxidative metabolism to oxypurinol, with no detectable plasma concentration of allopurinol after 5 hours post dosing. Approximately 12% of the allopurinol intravenous dose was excreted unchanged, 76% excreted as oxypurinol, and the remaining dose excreted as riboside conjugates in the urine. The rapid conversion of allopurinol to oxypurinol was not significantly different after repeated allopurinol dosing. ... Oxypurinol was primarily eliminated unchanged in urine by glomerular filtration and tubular reabsorption, with a net renal clearance of about 30 mL/min. For more Absorption, Distribution and Excretion (Complete) data for Allopurinol (13 total), please visit the HSDB record page. Metabolism / Metabolites Allopurinol is rapidly metabolized to the corresponding xanthine analog, oxipurinol (alloxanthine), which is also an inhibitor of xanthine oxidase enzyme. Both allopurinol and oxypurinol inhibit the action of this enzyme. Allopurinol and oxypurinol are also converted by the purine salvage pathway to their respective ribonucleotides. The effect of these ribonucleotides related to the hypouricemic action of allopurinol in humans is not fully elucidated to this date. These metabolites may act to inhibit de novo purine biosynthesis by inhibiting the enzyme, _amidophosphoribosyltransferase_. The ribonucleotides have not been found to be incorporated in DNA. Allopurinol and allopurinol sodium are rapidly metabolized by xanthine oxidase to oxypurinol, which is pharmacologically active. Rapid metabolism of allopurinol to oxypurinol does not seem to be affected substantially during multiple dosing. Pharmacokinetic parameters (eg, AUC, plasma elimination half-lives) of oxypurinol appear to be similar following oral administration of allopurinol and iv administration of allopurinol sodium. Both allopurinol and oxypurinol are conjugated and form their respective ribonucleosides. Allopurinol-1-riboside, a major metabolite of allopurinol, is commonly thought to be directly synthesized by purine nucleoside phosphorylase (PNP) in vivo. As this enzyme is otherwise believed to function in vivo primarily in the direction of nucleoside breakdown, we have determined by high performance liquid chromatography and a conventional chromatographic method the urinary metabolites of allopurinol in a child deficient of PNP. In this patient approximately 40% of urinary allopurinol metabolites consisted of allopurinol-1-riboside, thus proving the possibility of indirect formation of allopurinol-1-riboside via allopurinol-1-ribotide in vivo, catalysed by hypoxanthine guanine phosphoribosyltransferase (HGPRT) and a phosphatase. ... The major and active metabolite, oxypurinol, is detected in the circulation within 15 minutes of allopurinol administration. Oxypurinol concentrations are higher than those of the parent drug and accumulation occurs during long term administration. ...Oxypurinol is eliminated by the kidney and has a much longer elimination half-life than allopurinol. Oxypurinol accumulates in patients with renal dysfunction; hence allopurinol dosages should be adjusted in such patients. ... For more Metabolism/Metabolites (Complete) data for Allopurinol (7 total), please visit the HSDB record page. Biological Half-Life The plasma half-life of allopurinol is 1-2 hours, due to its rapid renal clearance. The half-lives of allopurinol and oxypurinol are about 1-3 hours and 18-30 hours, respectively, in patients with normal renal function and are increased in patients with renal impairment. Allopurinol is rapidly cleared from plasma with half-time of 2-3 hr, primarily by conversion to alloxanthine. Serum half-life of allopurinol is 39 min. |
| 毒性/毒理 (Toxicokinetics/TK) |
Interactions
Allopurinol inhibits enzymatic inactivation of 6-mercaptopurine by xanthine oxidase. Thus, when allopurinol is used ... with mercaptopurine or azathioprine, dosage of antineoplastic agent must be reduced to one fourth to one third of usual dose. Many drugs may increase serum urate concentrations, including most diuretics, pyrazinamide, diazoxide, alcohol, and mecamylamine. If these drugs are administered during allopurinol therapy, dosage of allopurinol may need to be increased. Concomitant administration of allopurinol with cyclophosphamide may increase the incidence of bone marrow depression as compared with cyclophosphamide alone, but the mechanism for this interaction is not known. However, results of a well-controlled study in patients with lymphoma have shown that concomitant use of allopurinol with cyclophosphamide, doxorubicin, bleomycin, procarbazine, and/or mechlorethamine did not increase the incidence of bone marrow depression in these patients. Incidence of rash occurring after the administration of ampicillin is unusually high in patients receiving allopurinol concomitantly. For more Interactions (Complete) data for Allopurinol (15 total), please visit the HSDB record page. |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
Antimetabolites; Antimetabolites, Antineoplastic; Enzyme Inhibitors; Gout Suppressants Allopurinol is indicated in the management of patients with signs and symptoms of primary or secondary gout (acute attacks, tophi, joint destruction, uric acid lithiasis, and/or nephropathy). /Included in US product label/ Allopurinol is indicated in the management of patients with leukemia, lymphoma and malignancies who are receiving cancer therapy which causes elevations of serum and urinary uric acid levels. Treatment with allopurinol should be discontinued when the potential for over production of uric acid is no longer present. /Included in US product label/ Allopurinol is indicated in the management of patients with recurrent calcium oxalate calculi whose daily uric acid excretion exceeds 800 mg/day in male patients and 750 mg/day in female patients. Therapy in such patients should be carefully assessed initially and reassessed periodically to determine in each case that treatment is beneficial and that the benefits outweigh the risks. /Included in US product label/ For more Therapeutic Uses (Complete) data for Allopurinol (9 total), please visit the HSDB record page. Drug Warnings Since allopurinol and oxypurinol are distributed into milk, allopurinol should be used with caution in nursing women. Results of early clinical studies and experience suggested that some allopurinol-induced adverse effects (eg, acute attacks of gout, rash) occurred in more than 1% of patients, but current experience suggests that adverse effects of the drug occur in less than 1% of patients. The reduced incidence in adverse effects observed with more recent experience may have resulted in part from initiating therapy with the drug more gradually and following current prescribing precautions and recommendations. The most common adverse effect of oral allopurinol is a pruritic maculopapular rash. Dermatitides of the exfoliative, urticarial, erythematous, eczematoid, hemorrhagic, and purpuric types have also occurred. Alopecia, fever, and malaise may also occur alone or in conjunction with dermatitis. In addition, severe furunculoses of the nose, cellulitis, and ichthyosis have been reported. The incidence of rash may be increased in patients with renal insufficiency. Skin reactions may be delayed and have been reported to occur as long as 2 years after initiating allopurinol therapy. Rarely, skin rash may be followed by severe hypersensitivity reactions which may sometimes be fatal. Some patients who have developed severe dermatitis have also developed cataracts (including a case of toxic cataracts), but the exact relationship between allopurinol and cataracts has not been established. Pruritus, onycholysis, and lichen planus have also occurred rarely in patients receiving allopurinol. Facial edema, sweating, and skin edema have also occurred rarely, but a causal relationship to the drug has not been established. Local injection site reactions have been reported in patients receiving allopurinol sodium iv. For more Drug Warnings (Complete) data for Allopurinol (28 total), please visit the HSDB record page. Pharmacodynamics Allopurinol decreases the production of uric acid by stopping the biochemical reactions that precede its formation. This process decreases urate and relieves the symptoms of gout, which may include painful tophi, joint pain, inflammation, redness, decreased range of motion, and swelling. |
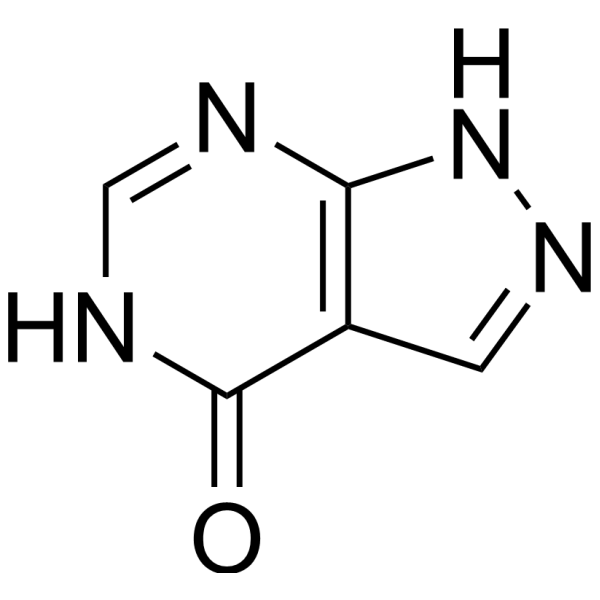
| 分子式 |
C5H4N4O
|
|---|---|
| 分子量 |
136.11146
|
| 精确质量 |
136.038
|
| CAS号 |
315-30-0
|
| 相关CAS号 |
Allopurinol sodium;17795-21-0;Allopurinol-d2;916979-34-5
|
| PubChem CID |
135401907
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.7±0.1 g/cm3
|
| 沸点 |
290.8ºC at 760 mmHg
|
| 熔点 |
350 ºC
|
| 闪点 |
129.7ºC
|
| 折射率 |
1.816
|
| LogP |
-1.46
|
| tPSA |
74.43
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
0
|
| 重原子数目 |
10
|
| 分子复杂度/Complexity |
190
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
OFCNXPDARWKPPY-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C5H4N4O/c10-5-3-1-8-9-4(3)6-2-7-5/h1-2H,(H2,6,7,8,9,10)
|
| 化学名 |
1,5-dihydropyrazolo[3,4-d]pyrimidin-4-one
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~14 mg/mL (~102.86 mM)
H2O : ~1 mg/mL (~7.35 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 3.33 mg/mL (24.47 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,将 100 μL 33.3 mg/mL 澄清 DMSO 储备液加入 900 μL 20% SBE-β-CD 生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 2 中的溶解度: ≥ 3.33 mg/mL (24.47 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 33.3 mg/mL 的澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 View More
配方 3 中的溶解度: ≥ 0.61 mg/mL (4.48 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 7.3470 mL | 36.7350 mL | 73.4700 mL | |
| 5 mM | 1.4694 mL | 7.3470 mL | 14.6940 mL | |
| 10 mM | 0.7347 mL | 3.6735 mL | 7.3470 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Treat-to-Target Serum Urate Versus Treat-to-Avoid Symptoms in Gout
CTID: NCT04875702
Phase: Phase 4 Status: Recruiting
Date: 2024-10-01
|
|
|