| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
| 靶点 |
ETA receptor
Endothelin A receptor (ET_A) (Ki = 0.011 nM, human; IC50 = 0.03 nM for ET-1 binding inhibition) [1][2] - Endothelin B receptor (ET_B) (Ki = 40 nM, human; >3600-fold lower affinity than ET_A) [1][2] - Nuclear factor erythroid 2-related factor 2 (Nrf2) (EC50 = 5 μM for nuclear translocation activation in hepatocytes) [2] |
|---|---|
| 体外研究 (In Vitro) |
Ambrisentan 仅在浓度高于 100 μM 时增加 P388/dx 细胞中的细胞内钙黄绿素荧光,而在 L-MDR1 细胞中则根本不表明 P-gp 抑制可忽略不计。 Ambrisentan 以浓度依赖性方式抑制这些组织中的特异性 [(125)I]ET-1 结合。安立生坦主要通过细胞色素 P450 (CYP) 3A4 进行氧化代谢,少量通过 CYP3A5 和 CYP2C19 进行。 Ambrisentan 不仅是 CYP3A4 的强诱导剂,而且还是 ABCB1 和 ABCG2 的强诱导剂。 Ambrisentan 对 PXR 活性也具有浓度依赖性影响,但由于未达到平台效应,因此无法计算 EC50 值。 Ambrisentan 在 IC50 纳摩尔范围内以浓度依赖性方式抑制特异性 [(125)I]ET-1 结合。 Ambrisentan 显着增加膀胱 [(125)I]ET-1 结合的解离常数,而不影响最大结合位点数量 (Bmax)。 Ambrisentan 似乎以竞争性和可逆的方式与膀胱 ET-1 受体结合。 Ambrisentan 是一种有效且安全的治疗方法,是对 PAH 治疗药物的宝贵补充。安布里生坦相对缺乏药物相互作用,每日一次给药并保证肝脏安全,比波生坦具有安全性和便利性优势。细胞测定:除非另有说明,对于每个BMEC实验,细胞被随机分为4组:(1)常氧载体对照(Nx-CTRL); (2)常氧处理; (3)缺氧(24小时)对照(Hx-CTRL)和(4)缺氧(24小时)治疗。如前所述,Nrf2 激活剂在任何缺氧暴露前 24 小时添加。细胞治疗是; Protandim (100 μg/mL)、醋甲唑胺 (125 μg/mL)、硝苯地平 (7 μg/mL) 或 Ambrisentan (40 μg/mL)。此外,一些细胞用 Nrf2 siRNA 处理。在这些实验中,添加了 siRNA 24药物治疗前 h。BMEC 24 小时缺氧暴露的基本原理是确保细胞在药物预处理(常氧条件下 24 小时)和 24 小时缺氧暴露期间保持被 siRNA 转染。数据收集自在三天内至少进行三次独立的细胞培养制备(n=9)。
Ambrisentan (BSF 208075; LU 208075)(安立生坦)是强效、高选择性ET_A受体拮抗剂,兼具Nrf2激活活性[1][2] - 在人肝星状细胞(HSCs)中,Ambrisentan(1-10 μM)剂量依赖性抑制TGF-β1诱导的增殖45-65%,通过阻断ET_A介导的Smad2/3磷酸化,下调纤维化标志物(α-SMA、I型胶原蛋白)35-50%[1] - 在人肝细胞(HepG2)中,Ambrisentan(5-20 μM)激活Nrf2核转位(EC50 = 5 μM),上调抗氧化基因(HO-1、NQO1)2.5-3.2倍,减少H2O2诱导的ROS生成40-55%[2] - 浓度高达100 μM时,对人支气管平滑肌细胞中ET_B介导的信号无显著影响[1] |
| 体内研究 (In Vivo) |
在Ambrisentan组中,肝脏羟脯氨酸含量显着低于对照组(分别为18.0 μg/g±6.1 μg/g vs 33.9 μg/g±13.5 μg/g肝脏,P=0.014)。通过天狼星红染色估计的肝纤维化和α-平滑肌肌动蛋白阳性区域(表明肝星状细胞活化)在安立生坦组中也显着降低(分别为 0.46%±0.18% vs 1.11%±0.28%,P=0.0003 ;和0.12%±0.08% vs 0.25%±0.11%,分别,P=0.047)。此外,Ambrisentan 组中原胶原-1 和金属蛋白酶组织抑制剂-1 (TIMP-1) 的肝脏 RNA 表达水平分别显着降低 60% 和 45%。肝脏中炎症、脂肪变性和内皮素相关 mRNA 表达在各组之间没有显着差异。 Ambrisentan 通过抑制肝星状细胞活化和减少 proollagen-1 和 TIMP-1 基因表达来减轻肝纤维化的进展。 Ambrisentan 不影响炎症或脂肪变性。
在非酒精性脂肪性肝炎(NASH)小鼠模型中,口服Ambrisentan(1-5 mg/kg/天,连续16周)剂量依赖性减轻肝纤维化(胶原蛋白沉积减少30-50%)和炎症(TNF-α/IL-6水平降低35-45%)[1] - 在急性高原病(AMS)大鼠模型中,口服Ambrisentan(10 mg/kg/天,连续3天)激活肺组织Nrf2信号,使HO-1表达增加2.8倍,减少肺氧化应激(MDA水平降低40%)[2] - 在NASH小鼠中,Ambrisentan(5 mg/kg/天)改善肝脂肪变性(甘油三酯含量减少35%),减轻肝损伤(ALT/AST水平降低25-30%)[1] |
| 酶活实验 |
ET_A/ET_B受体结合实验:制备表达人ET_A/ET_B的细胞膜制剂,与[¹²⁵I]-ET-1(0.1 nM)及不同浓度的Ambrisentan(0.0001-100 nM)在25°C孵育90分钟。在过量未标记ET-1存在下测定非特异性结合,过滤分离结合态配体,定量放射性强度以计算Ki值[1][2]
- Nrf2激活实验:HepG2细胞转染ARE-荧光素酶报告质粒,经Ambrisentan(1-20 μM)处理24小时。检测荧光素酶活性,评估Nrf2介导的转录激活[2] - Smad磷酸化实验:HSCs经Ambrisentan(1-10 μM)预处理1小时后,用TGF-β1(5 ng/mL)刺激6小时。Western blot检测并定量Smad2/3磷酸化水平[1] |
| 细胞实验 |
除非另有说明,每次 BMEC 实验的细胞被随机分配到四组:(1)常氧载体对照(Nx-CTRL); (2)常氧处理; (3)缺氧(24 h)对照(Hx-CTRL); (4)缺氧(24小时)处理。如前所述,在任何缺氧暴露前 24 小时添加 Nrf2 激活剂。 Protandim (100 μg/mL)、醋甲唑胺 (125 μg/mL)、硝苯地平 (7 μg/mL) 或 ambrisentan (40 μg/mL) 是细胞处理剂。此外,Nrf2 siRNA 还应用于一部分细胞。在这些测试中,在给药前 24 小时添加 siRNA。 BMEC 24小时缺氧暴露的目的是保证细胞在24小时缺氧暴露和药物预处理(常氧24小时)期间维持其siRNA转染。在不同的三天 (n=9),从至少三种不同的细胞培养制剂中收集数据 [2]。
肝星状细胞增殖实验:HSCs接种于96孔板,经Ambrisentan(1-10 μM)预处理1小时后,用TGF-β1(5 ng/mL)刺激72小时。MTT法测定细胞活力[1] - 抗氧化活性实验:HepG2细胞经Ambrisentan(5-20 μM)预处理24小时后,暴露于H2O2(100 μM)6小时。荧光探针染色定量细胞内ROS[2] - 纤维化标志物表达实验:HSCs经Ambrisentan(1-10 μM)联合TGF-β1(5 ng/mL)处理48小时。免疫荧光和Western blot检测α-SMA和I型胶原蛋白表达[1] |
| 动物实验 |
Mice: The experimental group consists of thirteen male FLS-ob/ob mice, weighing 42.88 g±1.74 g and aged 8 weeks. Male FLS-ob/ob mice are randomized at random to either the control (n = 5) or Ambrisentan (n = 8) group at 12 weeks or older. When a conscious animal has a gastric tube that is the right size, intragastric gavage is administered. Through the use of a gastric tube, ambrisentan (2.5 mg/kg daily) is given orally as a bolus every afternoon for four weeks. The group under control receives water treatment. The fourth week involves fasting the animals for four hours, drawing blood from the tail vein, and testing their blood glucose levels. Blood is extracted from the right ventricle and the animals are put to death after four weeks by injection with pentobarbital anesthesia. Plasma samples are kept at -80°C in a frozen state. The fat from the liver and viscera is then weighed, liquid nitrogen-snap frozen, and kept at -80°C for storage. Further liver specimens are embedded in paraffin and fixed in 10% buffered formalin for histological examination.
NASH mouse model: Male C57BL/6 mice were fed a high-fat/high-fructose diet for 16 weeks to induce NASH. Ambrisentan suspended in 0.5% CMC-Na was administered orally at 1, 3, 5 mg/kg/day throughout the diet period. Hepatic fibrosis, steatosis, and inflammation were evaluated [1] - Acute mountain sickness (AMS) rat model: Male Sprague-Dawley rats were exposed to hypobaric hypoxia (simulated 5000 m altitude) for 3 days to induce AMS. Ambrisentan (10 mg/kg/day) suspended in 0.5% CMC-Na was administered orally 1 day before and during hypoxia exposure. Pulmonary oxidative stress and Nrf2 signaling were analyzed [2] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Ambrisentan is rapidly absorbed with peak plasma concentrations occuring around 2 hours after oral administration. Cmax and AUC increase proportionally with dose across the therapeutic dosing range. Absolute oral bioavailability of ambrisentan is unknown. Absorption is not affected by food. Ambrisentan is primarily cleared by non-renal pathways. Along with its metabolites, ambrisentan is primarily found in the feces following hepatic and/or extra-hepatic metabolism. Approximately 22% of the administered dose is recovered in the urine following oral administration with 3.3% being unchanged ambrisentan. Ambrisentan has a low distribution into red blow cells, with a mean blood:plasma ratio of 0.57 and 0.61 in males and females, respectively. The mean oral clearance of ambrisentan was found to be 38 mL/min in healthy subjects and 19 mL/min in patients with pulmonary artery hypertension. Metabolism / Metabolites Ambrisentan is a metabolized primarily by uridine 5’-diphosphate glucuronosyltransferases (UGTs) 1A9S, 2B7S,1A3S to form ambrisentan glucuronide. Ambrisentan is also metabolized to a lesser extent by CYP3A4, CYP3A5 and CYP2C19 to form 4- hydroxymethyl ambrisentan which is further glucuronidated to 4-hydroxymethyl ambrisentan glucuronide. Biological Half-Life Ambrisentan has a terminal half-life of 15 hours. It is thought that steady state is achieved after around 4 days of repeat-dosing. Oral bioavailability: ~90% in humans; ~85% in rats after oral administration [1] - Elimination half-life: 15-19 hours in humans; 12.6 hours in rats [1] - Plasma protein binding: 94-98% in human plasma (concentration range: 0.1-10 μg/mL) [1] - Distribution: Volume of distribution (Vd) = 18 L/kg in humans, with extensive distribution to liver, lungs, and vascular tissues [1] - Metabolism/Excretion: Metabolized in the liver via glucuronidation; 70% of dose excreted as metabolites in urine, 20% in feces; <5% excreted unchanged [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Ambrisentan is associated with a low rate of serum aminotransferase elevations (0% to 3%) that in clinical trials was similar to the rate in placebo recipients. These elevations are usually mild (rarely above 3 times ULN), transient and not associated with symptoms. For these reasons, monthly monitoring of serum aminotransferase levels is no longer routinely recommended during ambrisentan therapy. There have also been no published reports of clinically apparent liver injury with jaundice associated with ambrisentan, but it has had limited general use. Other endothelin receptor antagonists (bosentan, sitaxsentan) have been linked to cases of acute liver injury, some of which have been severe. The onset of illness was usually within 1 to 6 months of starting bosentan and the enzyme pattern was typically hepatocellular or mixed. Immunoallergic features were usually not present and autoantibodies absent or present in low titer. Sitaxsentan was linked to several cases of fatal acute liver failure, for which reason it was not approved in the United States and was later withdrawn from use elsewhere. Ambrisentan has not been linked to similar cases and its chemical structure is sufficiently different to suggest lack of cross sensitivity to this complication. Likelihood score: E (unlikely cause of clinically apparent liver injury). Protein Binding Ambrisentan is 99% plasma protein bound, primarily to albumin (96.5%) and to a lesser degree alpha1-acid glycoprotein. Acute toxicity: Oral LD50 > 1000 mg/kg in rats; >800 mg/kg in mice [1] - Subchronic toxicity (16-week oral administration in NASH mice): No significant hepatotoxicity or nephrotoxicity at doses up to 5 mg/kg/day; no changes in body weight or hematological parameters [1] - In HepG2 cells, no significant cytotoxicity at concentrations up to 50 μM [2] - Drug-drug interactions: No significant inhibition of CYP450 enzymes; minimal interaction with common medications (e.g., warfarin, statins) [1] |
| 参考文献 | |
| 其他信息 |
Pharmacodynamics
Ambrisentan 10 mg daily had no significant effect on the QTc interval, whereas a 40 mg daily dose of ambrisentan increased mean QTc at tmax by 5 ms with an upper 95% confidence limit of 9 ms. Significant QTc prolongation is not expected in patients taking ambrisentan without concomitant metabolic inhibitors. Plasma concentrations of B-type natriuretic peptide (BNP) in patients who received ambrisentan for 12 weeks were significantly decreased. Two Phase III placebo-controlled studies demonstrated a decrease in BNP plasma concentrations by 29% in the 2.5 mg group, 30% in the 5 mg group, and 45% in the 10 mg group (p < 0.001 for each dose group) and an increase by 11% in the placebo group. Ambrisentan (BSF 208075; LU 208075) is a potent, highly selective ET_A receptor antagonist approved for the treatment of pulmonary arterial hypertension (PAH), with additional antifibrotic and antioxidant properties [1][2] - Its core mechanisms include two key actions: blocking ET_A-mediated signaling to inhibit vascular smooth muscle proliferation and fibrosis, and activating Nrf2 to enhance antioxidant defense and reduce oxidative stress [1][2] - Therapeutic applications include treatment of PAH, and investigation for NASH-related hepatic fibrosis and acute mountain sickness (AMS) via antifibrotic and antioxidant effects [1][2] - High selectivity for ET_A over ET_B minimizes side effects such as edema and gastrointestinal disturbances associated with non-selective endothelin receptor antagonists [1] - Long elimination half-life in humans supports once-daily oral dosing, improving patient adherence [1] |
| 分子式 |
C22H22N2O4
|
|---|---|
| 分子量 |
378.42
|
| 精确质量 |
378.158
|
| 元素分析 |
C, 69.83; H, 5.86; N, 7.40; O, 16.91
|
| CAS号 |
177036-94-1
|
| 相关CAS号 |
Ambrisentan sodium; 1386915-48-5; Ambrisentan-d10; 1046116-27-1
|
| PubChem CID |
6918493
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.228g/cm3
|
| 沸点 |
551.1ºC at 760mmHg
|
| 熔点 |
>150°C (dec.)
|
| 闪点 |
287.1ºC
|
| 蒸汽压 |
5.56E-13mmHg at 25°C
|
| 折射率 |
1.593
|
| LogP |
3.515
|
| tPSA |
81.54
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
28
|
| 分子复杂度/Complexity |
475
|
| 定义原子立体中心数目 |
1
|
| SMILES |
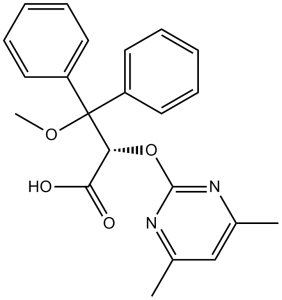
O=C([C@H](C(OC)(C1=CC=CC=C1)C2=CC=CC=C2)OC3=NC(C)=CC(C)=N3)O
|
| InChi Key |
OUJTZYPIHDYQMC-LJQANCHMSA-N
|
| InChi Code |
InChI=1S/C22H22N2O4/c1-15-14-16(2)24-21(23-15)28-19(20(25)26)22(27-3,17-10-6-4-7-11-17)18-12-8-5-9-13-18/h4-14,19H,1-3H3,(H,25,26)/t19-/m1/s1
|
| 化学名 |
(2S)-2-(4,6-dimethylpyrimidin-2-yl)oxy-3-methoxy-3,3-diphenylpropanoic acid
|
| 别名 |
LU-208075; BSF-208075; BSF208075; LU208075; BSF 208075; Letairis; Volibris; LU-208075; BSF-208075; (S)-2-(4,6-Dimethylpyrimidin-2-yloxy)-3-methoxy-3,3-diphenylpropanoic acid; (S)-2-((4,6-Dimethylpyrimidin-2-yl)oxy)-3-methoxy-3,3-diphenylpropanoic acid; LU 208075; trade name Letairis; Volibris; pulmonext
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.61 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (6.61 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 View More
配方 3 中的溶解度: ≥ 0.71 mg/mL (1.88 mM) (饱和度未知) in 10% EtOH + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: ≥ 0.71 mg/mL (1.88 mM) in 10% EtOH + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,您可以将 100 μL 7.1 mg/mL 澄清乙醇储备液添加到 900 μL 20% SBE-β-CD 生理盐水溶液中并充分混合。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 5 中的溶解度: 10% EtOH + 90% Corn Oil 配方 6 中的溶解度: 12.5 mg/mL (33.03 mM) in 0.5% CMC-Na/saline water (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6426 mL | 13.2128 mL | 26.4257 mL | |
| 5 mM | 0.5285 mL | 2.6426 mL | 5.2851 mL | |
| 10 mM | 0.2643 mL | 1.3213 mL | 2.6426 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05437224 | Completed | Drug: Ambrisentan | Pulmonary Arterial Hypertension | RenJi Hospital | December 18, 2018 | Phase 3 |
| NCT01330108 | Completed | Drug: ambrisentan | Pulmonary Arterial Hypertension | University of Alabama at Birmingham | May 2011 | Phase 4 |
| NCT01072669 | Completed | Drug: ambrisentan | Ischemia | Soumya Chatterjee | February 2010 | Not Applicable |
| NCT01224210 | Completed | Drug: Ambrisentan | Portopulmonary Hypertension | Tufts Medical Center | March 2010 | Phase 3 |
|
|
|