| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
Androgen receptor (AR) (IC50: 16 nM in binding assay) [1]
|
|---|---|
| 体外研究 (In Vitro) |
在放射性配体结合实验中,aparalutamide (ARN-509) 表现出对 GABAA 受体的低微摩尔亲和力 (IC50 3 μM),表明它可能在抑制水平上拮抗 GABAA [1]。 apelutamide 强烈抑制 AR 配体结合结构域,阻断 AR 基因靶标的转录、DNA 结合和雄激素受体 (AR) 的核转位 [2]。
- AR结合抑制:阿帕他胺(ARN-509)对AR具有高亲和力,在放射性配体结合实验中IC50为16 nM。免疫荧光显微镜显示,其阻断雄激素诱导的AR核转位和DNA结合。Western blot分析显示,在LNCaP前列腺癌细胞中,阿帕他胺剂量依赖性抑制AR响应基因(如PSA和TMPRSS2)的表达[1]。 - 抗增殖活性:阿帕他胺抑制AR阳性前列腺癌细胞系(LNCaP、22Rv1)的生长,IC50为0.3–0.5 μM。集落形成实验显示,与对照组相比,克隆形成存活率降低70–80%[1]。 - 诱导凋亡:流式细胞术分析显示,阿帕他胺(1–2 μM)使LNCaP细胞中膜联蛋白V阳性凋亡细胞增加25–30%,同时伴随caspase-3和PARP的切割[1]。 |
| 体内研究 (In Vivo) |
Apalutamide (ARN-509) 由于其血浆半衰期长、口服生物利用度优异且全身清除率低,支持小鼠和狗每天一次口服治疗。在重复给药实验中,阿帕他胺的稳态血浆水平上升,这与其延长的终末半衰期一致。这导致 C24 小时水平较高,峰谷比较低(比率:2.5)。将 Apalutamide 以 1、10 或 30 mg/kg/天的剂量给予患有 LNCaP/AR 异种移植肿瘤的去势雄性小鼠。第 28 天,用 Apalutamide(30 mg/kg/天)治疗的 20 只小鼠中,有 13 只观察到肿瘤体积减少 >50%,而用 MDV3100(30 mg/kg/天)治疗的 19 只小鼠中,有 19 只小鼠的肿瘤体积减少了 50% 以上。只有 3[1]。
- 异种移植模型中的肿瘤生长抑制:携带LNCaP肿瘤的裸鼠接受阿帕他胺(20–50 mg/kg,口服每日一次)治疗。28天后,50 mg/kg组肿瘤体积较对照组缩小65%。免疫组化显示,治疗组肿瘤中Ki-67染色减少,cleaved caspase-3增加[1]。 - 高危NM-CRPC患者的疗效:在一项II期研究中,阿帕他胺(240 mg/天)显著延迟PSA倍增时间≤10个月患者的影像学进展。治疗组中位影像学无进展生存期为24.8个月,对照组为16.2个月(HR=0.45,P<0.001)。89%的治疗组患者出现PSA应答(≥50%下降)[2]。 |
| 酶活实验 |
配体结合研究[1]
全细胞LNCaP/AR:在LNCaP/AR(密码子转换)(LNCaP/AR(cs))(含有外源野生型AR和内源性突变型AR(T877A)的混合物)和在补充10%胎牛血清(FBS)的Iscove或RPMI培养基中繁殖的细胞中进行全细胞竞争性结合测定,或在用10%炭剥离的右旋糖酐处理的胎牛血清进行测定期间进行。将细胞与18F-FDHT预孵育,加入浓度增加(1pM至1μM)的冷竞争对手,并根据已公布的程序进行测定,以测量18F-FDHT的特异性摄取(4)。使用具有最小二乘曲线拟合的单位点结合模型测定IC50值,R2>0.99。 全细胞提取物MDA-MB-453细胞:在含有20mM HEPES、4mM l-谷氨酰胺、10μg/mL人胰岛素、10%FBS和20μg/mL庆大霉素的RPMI 1640中培养MDA-MB-45 3细胞(内源性野生型AR;ATCC:HTB131)。在达到90%汇合后,收获细胞,重悬于TEGM(10mM Tris-HCl pH 7.2,1mM EDTA,10%甘油,1mM-巯基乙醇,10mM钼酸钠),并在液氮中冷冻在含有4x107个细胞/mL的10mL等分试样中。结合反应(60uL)在TEGM的96孔板中进行,通常含有24μL细胞裂解物、1.2nM 3H-R1881(Perkin-Elmer)和10-10-10-4M各自的竞争性配体。反应物在4°C下孵育过夜。使用Unifilter-96 GF/C滤板(Perkin-Elmer)通过超滤分离结合和未结合的配体。结合的3H-R1881在30uL/孔Microscint-20中洗脱,并使用顶部计数进行定量。Ki根据Cheng Prusoff(5)计算为Ki=IC50/(1+([3H-R1881]/Kd)) 体外:根据已公布的程序(4),使用竞争性测定试剂盒(绿色)测定ARN-509对大鼠AR配体结合域(LBD)、人孕酮受体(PR)LBD和全长人雌激素受体α(ER) 和人糖皮质激素受体(GR)。每种激素剂量一式三份,根据平均值的标准误差(SEM)计算相对误差,并使用R2>0.8的单结合位点竞争模型(Prism统计分析软件包)拟合结合曲线。以SEM<平均logIC50值的0.3 log单位进行多次实验。Ki值计算为SEM实验的平均值,结合亲和力报告为相对于该受体的紧密结合配体对照的百分比。 AR结合实验:将重组人AR与[³H]-R1881(合成雄激素)及梯度浓度的阿帕他胺在含10%甘油和蛋白酶抑制剂的缓冲液中孵育。通过葡聚糖包被活性炭沉淀分离结合的放射性配体,非线性回归分析确定IC50[1]。 |
| 细胞实验 |
抗增殖活性测定[1]
在不含酚红的RPMI 1640(含5%CSS)中将胰蛋白酶化的VCaP细胞调节至每毫升100000个细胞的浓度,并以16µL等分试样的形式分配到CellBIND 384孔板中。将细胞孵育48小时,之后将16µL体积的配体添加到RPMI培养基中。对于拮抗剂模式测定,在同样含有30pM R1881(最终[R1881]=15pM)的培养基中稀释配体。培养7天后,加入16µL CellTiter Glo发光细胞活力测定,并测量相对发光单位(RLU) 在激动剂模式测定中,样品的生存率百分比计算为:生存率百分比=[RLU样品RLU培养基不含细胞]/[RLU-DMSO处理的细胞RLU培养基不含细胞]。在拮抗剂模式测定中,样品的生存率百分比计算为:生存率百分比=[RLU样品RLU VCaP不含R1881]/[RLU R1881处理的细胞-RLU VCaP无R1881]。 - AR核转位实验:用阿帕他胺(0.1–1 μM)处理LNCaP细胞2小时,固定后用抗AR抗体染色。共聚焦显微镜显示,剂量依赖性地将AR保留在细胞质中,1 μM时抑制率>90%[1]。 - PSA分泌实验:用阿帕他胺(0.5–2 μM)处理C4-2B前列腺癌细胞48小时。ELISA显示,分泌的PSA水平较对照组降低60–80%[1]。 |
| 动物实验 |
Dissolved in 15% Vitamin E-TPGS and 65% of a 0.5% w/v CMC solution in 20 mM citrate buffer (pH 4.0), and diluted in saline; 30 mg/kg/day; oral administration
Castrate male immunodeficient mice harboring LNCaP/AR-luc xenograft tumors In vivo pharmacodynamic studies [1] Pharmacodynamic studies with LNCaP/AR-luc xenografts in castrate male SCID mice were performed as previously described (4). Formalin-fixed, paraffin-embedded tissue was processed and stained for hematoxylin and eosin (H&E), terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) or immunohistochemistry (IHC) for Ki67 as previously described (4). In vivo luciferase imaging of mice with LNCaP/AR-luc xenografts was performed according to published methods (4), and data analyzed using Living Image 2.30 software. As part of an Investigational New Drug (IND)-enabling toxicity and toxicokinetic study, ARN-509 was administered to male beagle dogs (aged 6 to 7 months, with body weights ranging from 9.3 to 11.2 kg), by Covance Laboratories Inc., in accordance with the United States Food and Drug Administration (FDA) Good Laboratory Practice (GLP) Regulations. ARN-509 was administered daily for 28 days at 0 mg/kg (5 dogs) or 10 mg/kg (4 dogs) by oral gavage (po). ARN-509 (3.33 mg/mL) was formulated as a suspension in labrasol (10% v/v), lactic acid (10% v/v) and soybean oil (10% v/v) and brought up to volume with 50mM phosphate buffer. The placebo oral formulation contained 0 mg/mL ARN-509. Mouse and dog pharmacokinetics [1] Mouse (male CD-1) or beagle dog (Charles River) plasma samples (25 µL) were combined with 100 µL of acetonitrile:methanol:acetic acid, 1:1:0.001, v/v/v containing 500 ng/mL ARN-509-d3 as an internal standard. Precipitated proteins were removed by centrifugation at 1,500 g for 20 minutes at 5°C. Supernatant (50 µL) was diluted with 400 µL of 2:1 water:acetonitrile. ARN-509 concentrations were quantified using the LC-MS/MS method below. - Xenograft tumor model: LNCaP cells (5×10⁶) were implanted subcutaneously into male nude mice. Once tumors reached 100–150 mm³, mice were randomized to receive Apalutamide (20 or 50 mg/kg) suspended in 0.5% methylcellulose via oral gavage daily for 28 days. Tumor volumes were measured twice weekly using calipers [1]. - Toxicity evaluation in mice: C57BL/6 mice received Apalutamide (100–200 mg/kg) orally for 14 days. Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were measured, showing no significant elevation compared to control [1]. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Mean absolute oral bioavailability was approximately 100%. The median time to achieve peak plasma concentration (tmax) was 2 hours (range: 1 to 5 hours). The major active metabolite N-desmethyl apalutamide Cmax was 5.9 mcg/mL (1.0) and AUC was 124 mcg·h/mL (23) at steady-state after the recommended dosage. Administration of apalutamide to healthy subjects under fasting conditions and with a high-fat meal (approximately 500 to 600 fat calories, 250 carbohydrate calories, and 150 protein calories) resulted in no clinically relevant changes in Cmax and AUC. The median time to reach tmax was delayed approximately 2 hours with food. Following administration of the recommended dosage, apalutamide steady-state was achieved after 4 weeks and the mean accumulation ratio was approximately 5-fold. Apalutamide Cmax was 6.0 mcg/mL (1.7) and AUC was 100 mcg·h/mL (32) at steady-state. Daily fluctuations in apalutamide plasma concentrations were low, with the mean peak-to-trough ratio of 1.63. Oral administration of four 60 mg apalutamide tablets dispersed in applesauce resulted in no clinically relevant changes in Cmax and AUC compared to the administration of four intact 60 mg tablets under fasting conditions. Apalutamide and its main active metabolite are subject to both renal and focal elimination. Up to 70 days following a single oral administration of radiolabeled apalutamide, 65% of the dose was recovered in urine (1.2% of dose as unchanged apalutamide and 2.7% as N-desmethyl apalutamide) and 24% was recovered in feces (1.5% of dose as unchanged apalutamide and 2% as N-desmethyl apalutamide). The mean apparent volume of distribution at steady state of apalutamide was approximately 276 L. The CL/F of apalutamide was 1.3 L/h after single dosing and increased to 2.0 L/h at steady-state after once-daily dosing likely due to CYP3A4 auto-induction. The auto-induction effect likely reached its maximum at the recommended dosage because exposure to apalutamide across the dose range of 30 to 480 mg is dose-proportional. Metabolism / Metabolites Metabolism is the main route of elimination of apalutamide. Apalutamide is primarily metabolized by CYP2C8 and CYP3A4 to form active metabolite, N-desmethyl apalutamide. The contribution of CYP2C8 and CYP3A4 in the metabolism of apalutamide is estimated to be 58% and 13% following single dose but changes to 40% and 37%, respectively at steady-state. The auto-induction of CYP3A4-mediated metabolism by apalutamide may explain the increase in CYP3A4 enzymatic activity at steady-state. Biological Half-Life The mean effective half-life for apalutamide in patients with NM-CRPC was approximately 3 days at steady-state. - Oral bioavailability: Apalutamide exhibited high oral bioavailability (89%) in rats, with peak plasma concentrations (Cmax) of 1.2 μg/mL achieved within 1 hour after dosing [1]. - Plasma protein binding: Plasma protein binding was >96% in human serum, primarily to albumin and α1-acid glycoprotein [1]. - Metabolism: The drug was metabolized in the liver via CYP2C8 and CYP3A4, with N-demethylation as the major pathway. The active metabolite N-desmethyl-apalutamide showed similar AR inhibitory activity (IC50=20 nM) [1]. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In prelicensure controlled trials of apalutamide, serum aminotransferase elevations were uncommon and generally transient and mild, not requiring dose modification. Clinically apparent liver injury with jaundice attributable to apalutamide was not reported in the preregistration trials and is not mentioned as an adverse event in the product label. Since the approval and general clinical use of apalutamide, there have been no publications or descriptions of the clinical features of hepatotoxicity with jaundice associated with its use. The first and second generation androgen receptor blockers, flutamide, nilutamide, and bicalutamide, have all been linked to instances of hepatitis-like liver injury with jaundice that can be severe and even fatal. However, such cases have not been described with apalutamide and other third generation androgen receptor antagonists. Thus, clinically apparent liver injury due to apalutamide must be rare, if it occurs at all. Likelihood score: E (unlikely cause of clinically apparent liver injury). Protein Binding Apalutamide was 96% and N-desmethyl apalutamide was 95% bound to plasma proteins with no concentration dependency. - Clinical safety profile: In phase 2 trials, Apalutamide (240 mg/day) was generally well-tolerated. Common adverse events included rash (21%), fatigue (18%), and hypertension (12%). No grade 3/4 hepatotoxicity or QT prolongation was observed [2]. - Preclinical toxicity: No significant adverse effects were noted in rat and dog toxicology studies at doses up to 200 mg/kg/day. Histopathological examination revealed no evidence of liver or kidney damage [1]. |
| 参考文献 |
|
| 其他信息 |
Pharmacodynamics
In androgen receptors (AR)-overexpressing LNCaP cells, apaludatamide was reported to have a 7 to 10-fold greater affinity to the AR than bicalutamide. Additionally, apalutamide still possesses total antagonistic activity in AR-overexpressing cell lines with bicalutamide-resistance mutations such as T878A and W741C. In castrate mice with LNCaP/AR(cs) tumors, apalutamide produced tumor regression (defined by >50% regression in tumor volume) in 8 mice compared to only 1 for bicalutamide. The apalutamide-treated tumors also have a 60% decrease in proliferative index and a 10-fold increase in apoptotic rate compared with vehicle. In an open-label, uncontrolled, multicenter, single-arm dedicated QT study in 45 patients with CRPC, an exposure-QT analysis suggested a concentration-dependent increase in QTcF for apalutamide and its active metabolite. Apalutamide demonstrated antitumor activity in the mouse xenograft models of prostate cancer, where it decreased tumor cell proliferation and reduced tumor volume. - Mechanism of action: Apalutamide acts as a competitive AR antagonist, blocking androgen binding, nuclear translocation, and transcriptional activity. It also induces apoptosis in AR-dependent prostate cancer cells through activation of caspase pathways [1]. - Clinical indication: Approved for treatment of nonmetastatic castration-resistant prostate cancer (NM-CRPC) and metastatic castration-sensitive prostate cancer (mCSPC) in combination with androgen deprivation therapy (ADT) [2]. - Resistance mechanisms: Emerging data suggest that Apalutamide resistance may involve AR gene amplification or mutations in the ligand-binding domain. Combination strategies with other targeted agents (e.g., PI3K inhibitors) are being explored [1]. Apalutamide is a potent androgen receptor (AR) antagonist that selectively binds to the ligand-binding domain of AR and blocks AR nuclear translocation or binding to androgen response elements. It has been used in trials studying the treatment of Prostate Cancer, Hepatic Impairment, Prostatic Neoplasms, Castration-Resistant Prostate Cancer, and Prostatic Neoplasms, Castration-Resistant, among others. Exerting an antitumor action, apalutamide blocking the effect of androgens that promote tumor growth. It targets the AR ligand-binding domain and prevents AR nuclear translocation, DNA binding, and transcription of AR gene targets in prostate tumors. In mice bearing human CRPC xenograft models, apalutamide treatment produced tumor regressions in a dose-dependent manner that was more effective than that of [DB01128] or [DB08899]. Unlike bicalutamide, apalutamide antagonized AR-mediated signaling in AR overexpressing human CRPC cell lines. Androgen-deprivation therapy, or hormone therapy, can be used as part of maintenance therapy for patients with non-metastatic prostate cancer. Although most patients achieve therapeutic responses at the initial hormone therapy, many patients progress to non-metastatic castration-resistant (resistance to hormone therapy) prostate cancer which is the second-most common cause of cancer-related deaths in American males. Castration-resistant prostate cancer is often incurable, which poses significant clinical challenges for patients. Approximately 10 to 20 % of prostate cancer cases are castration-resistant, and up to 16% of these patients show no evidence of cancer metastasis at the time of castration-resistant diagnosis. Higher prostate-specific antigen (PSA) and shorter PSA doubling time (PSA DT) are associated with a higher risk for metastases and death. In a phase-2 multicenter open-label study, 89% of patients with non-metastatic, castration-resistant prostate cancer had ≥50% PSA decline at week 12 of apalutamide treatment. In a randomized trial, the median metastasis-free survival for patients taking apalutamide was 40.5 months compared to 16.2 months for patients taking a placebo. Apalutamide displayed good tolerability and safety profile in clinical studies. Apalutamide was approved in February 2018 by the FDA as Erleada for the treatment of patients with non-metastatic prostate cancer that is resistant to treatment with hormone therapy (castration-resistant). It is available as oral tablets. Apalutamide is the first FDA-approved treatment for non-metastatic, castration-resistant prostate cancer. Apalutamide is a third generation, oral nonsteroidal antiandrogen used to treat nonmetastatic castration-resistant prostate cancer. Apalutamide is associated with a low rate of serum enzyme elevation during therapy but has not been linked to cases of clinically apparent liver injury with jaundice. Apalutamide is a small molecule and androgen receptor (AR) antagonist with potential antineoplastic activity. Apalutamide binds to AR in target tissues thereby preventing androgen-induced receptor activation and facilitating the formation of inactive complexes that cannot be translocated to the nucleus. This prevents binding to and transcription of AR-responsive genes. This ultimately inhibits the expression of genes that regulate prostate cancer cell proliferation and may lead to an inhibition of cell growth in AR-expressing tumor cells. APALUTAMIDE is a small molecule drug with a maximum clinical trial phase of IV (across all indications) that was first approved in 2018 and has 6 approved and 2 investigational indications. |
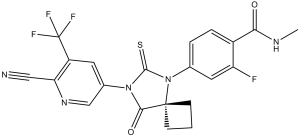
| 分子式 |
C21H15F4N5O2S
|
|---|---|
| 分子量 |
477.43
|
| 精确质量 |
477.088
|
| 元素分析 |
C, 52.83; H, 3.17; F, 15.92; N, 14.67; O, 6.70; S, 6.72
|
| CAS号 |
956104-40-8
|
| 相关CAS号 |
Apalutamide-d4;1638885-65-0;Apalutamide-d3;1638885-61-6;Apalutamide-13C,d3; 2376466-25-8 (acetate); 1505451-73-9 (ethanol); 1505451-74-0 (hydrate); 956104-40-8; 1505451-77-3 (DMSO)
|
| PubChem CID |
24872560
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.6±0.1 g/cm3
|
| 折射率 |
1.659
|
| LogP |
1.3
|
| tPSA |
121.42
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
9
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
33
|
| 分子复杂度/Complexity |
886
|
| 定义原子立体中心数目 |
0
|
| SMILES |
N#CC1C(C(F)(F)F)=CC(N2C(=S)N(C3C=C(F)C(C(NC)=O)=CC=3)C3(CCC3)C2=O)=CN=1
|
| InChi Key |
HJBWBFZLDZWPHF-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C21H15F4N5O2S/c1-27-17(31)13-4-3-11(8-15(13)22)30-19(33)29(18(32)20(30)5-2-6-20)12-7-14(21(23,24)25)16(9-26)28-10-12/h3-4,7-8,10H,2,5-6H2,1H3,(H,27,31)
|
| 化学名 |
4-(7-(6-cyano-5-(trifluoromethyl)pyridin-3-yl)-8-oxo-6-thioxo-5,7-diazaspiro[3.4]octan-5-yl)-2-fluoro-N-methylbenzamide
|
| 别名 |
JNJ56021927; ARN509; JNJ-56021927; ARN 509; JNJ 56021927; ARN-509; Apalutamide; Brand name: Erleada
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (4.36 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (4.36 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL 澄清 DMSO 储备液添加到 900 μL 玉米油中并混合均匀。 View More
配方 3 中的溶解度: 0.5% CMC, pH4.0:14 mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0945 mL | 10.4727 mL | 20.9455 mL | |
| 5 mM | 0.4189 mL | 2.0945 mL | 4.1891 mL | |
| 10 mM | 0.2095 mL | 1.0473 mL | 2.0945 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
 ARN-509 activityin vitroin human prostate-cancer cells.Cancer Res.2012 Mar 15;72(6):1494-503. |
|---|
 ARN-509impairs AR nuclear-localization and inhibits DNA-binding.Cancer Res.2012 Mar 15;72(6):1494-503. |
 ARN-509is active in models of castration-resistant prostate cancer.Cancer Res.2012 Mar 15;72(6):1494-503. |
 ARN-509achieves similar efficacy with lower steady-state plasma-levels than MDV3100 in LNCaP/AR xenograft models of castration-resistant prostate cancer.Cancer Res.2012 Mar 15;72(6):1494-503. |
|---|
 ARN-509induces castrate-like changes in dog prostate and epididymis.Cancer Res.2012 Mar 15;72(6):1494-503. |