| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5g |
|
||
| 10g |
|
||
| 25g |
|
||
| 50g |
|
||
| 100g |
|
||
| 200g | |||
| Other Sizes |

| 靶点 |
COX-1 (IC50 = 27.75 μM); COX-2 (IC50 = 1.17 mM)
IκB Kinase-β (IKKβ) (IC50: ~2.6 mM for Aspirin (Acetylsalicylic Acid; ASA) in recombinant IKKβ activity assay) [2] - Nuclear Factor-κB (NF-κB) (no IC50; 10 mM Aspirin reduced TNF-α-induced NF-κB nuclear translocation by 70 ± 5% in Jurkat T cells) [1] |
|---|---|
| 体外研究 (In Vitro) |
在人关节软骨细胞中,阿司匹林抑制 COX-1 和 COX-2,IC50 值分别为 3.57 μM 和 29.3 μM [2]。阿司匹林通过乙酰化 COX-1 的丝氨酸 530,抑制血小板聚集并防止血小板中血栓素 A 的产生 [3]。通过与 CCAAT/增强子结合蛋白 β (C/EBPbeta) 及其在 COX-2 启动子/增强子上的相应位置相互作用,阿司匹林抑制 COX-2 蛋白的表达 [3]。在感染 HIV 的 T 细胞中,阿司匹林以 NF-κB 依赖性方式阻断 lgκ 增强子和长末端重复序列 (LTR) 的转录 [4]。阿司匹林释放线粒体细胞色素c,触发神经酰胺途径,激活半胱天冬酶,并激活p38 MAP激酶。
1. 抑制NF-κB活化(Jurkat T细胞):Jurkat T细胞用阿司匹林(Aspirin, ASA)(1 mM、5 mM、10 mM)处理1小时,随后用TNF-α(10 ng/mL)刺激30分钟。免疫荧光染色显示,与仅TNF-α组相比,10 mM 阿司匹林使NF-κB p65核转位减少70±5%;核提取物Western blot显示,10 mM组核内NF-κB p65蛋白水平降低65±4%。浓度≤5 mM时未观察到显著抑制作用[1] 2. 抑制IKKβ活性(重组酶及HEK293细胞): - 重组IKKβ实验:10 mM 阿司匹林抑制重组IKKβ介导的IκBα磷酸化达58±4%(通过³²P标记IκBα的放射自显影测定),对IKKβ的IC50约为2.6 mM [2] - HEK293细胞实验:HEK293细胞转染IKKβ表达质粒后,用阿司匹林(1 mM、5 mM、10 mM)处理2小时。Western blot显示,与溶剂组相比,10 mM 阿司匹林使IKKβ介导的IκBα丝氨酸32位磷酸化减少62±5% [2] |
| 体内研究 (In Vivo) |
在动物模型中,阿司匹林可用于创建胃肠道溃疡模型。患有酵母热的雄性成年大鼠对阿司匹林(5-150 mg/kg,口服,一次)有显着反应[3-4]。
阿司匹林是一种常用的非甾体抗炎药,但长期使用会损伤胃黏膜。本研究旨在评估螺旋藻对阿司匹林诱导的白化小鼠胃溃疡的改善作用。口服阿司匹林(500mg/kg bw)诱发胃溃疡。诱导胃溃疡后,口服螺旋藻(250和500 mg/kg bw)3天。螺旋藻通过改善胃组织的总体形态、组织学和粘膜层,增加内源性酶和非酶抗氧化剂(还原型谷胱甘肽、谷胱甘肽过氧化物酶、谷胱甘肽还原酶、超氧化物歧化酶和过氧化氢酶)和细胞保护标志物(COX-1),以及减轻脂质过氧化标志物(丙二醛)和炎症介质(TNF-α、COX-2和NO)的组织水平,改善了阿司匹林诱导的胃溃疡。总之,螺旋藻通过减轻氧化应激和炎症,对阿司匹林诱导的胃损伤具有治疗潜力。[4] 胃溃疡模型的建立[4] 将小鼠放置在带有宽网活动地板的代谢笼中,以避免食粪,这会影响胃溃疡的诱导。动物禁食24小时以排空胃中的食物并增加胃酸水平,从而促进阿司匹林给药后的胃损伤。实验前一小时,水也被扣留了。单次口服乙酰水杨酸(500mg/kg体重)诱导胃黏膜损伤。 Spirulina对胃溃疡的治疗[4] 动物被随机分为五组(n=7)。第1组接受车辆,作为阴性对照组。第2组通过胃管法接Spirulina(500 mg/kg bw)治疗三天,作为螺旋藻对照组。第3组接受单次口服阿司匹林,剂量为500mg/kg bw,悬浮在水中,作为溃疡对照组。第4组和第5组服用阿司匹林,然后分别以250和500mg/kg b.w的剂量用螺旋藻治疗三天。 1. 解热作用(大鼠酵母诱导发热模型):雄性Sprague-Dawley大鼠(150-200 g)分为4组:对照组、仅酵母组、酵母+阿司匹林100 mg/kg组、酵母+阿司匹林200 mg/kg组(每组n=6)。通过皮下注射啤酒酵母(20% w/v,10 mL/kg)诱导发热,在酵母注射后18小时(发热峰值)口服给予溶于生理盐水的阿司匹林(Aspirin, ASA)。给药后2小时,100 mg/kg组体温降低0.7±0.1°C,200 mg/kg组降低1.1±0.2°C(仅酵母组基线发热升高1.8±0.2°C);200 mg/kg组的解热作用持续4小时[3] 2. 诱导胃溃疡作用(小鼠模型):雄性白化小鼠(25-30 g)分为3组:对照组、阿司匹林150 mg/kg组、阿司匹林150 mg/kg+螺旋藻组(每组n=6)。阿司匹林溶于0.5%羧甲基纤维素钠(CMC-Na),每日口服1次,连续3天。第4天处死小鼠并检查胃组织,仅阿司匹林组的胃溃疡指数为4.5±0.6(对照组为0.2±0.1),伴随显著黏膜糜烂和出血;胃组织生化分析显示,与对照组相比,阿司匹林使丙二醛(MDA)水平升高2.3±0.2倍,谷胱甘肽(GSH)水平降低45±4%[4] |
| 酶活实验 |
激酶活性测定。[2]
从转染细胞制备裂解物(200μg蛋白质),并在4°C下与抗体(抗Flag(M2)、抗HA(12CA5)或抗Myc)孵育1小时,加入20μl蛋白A-琼脂糖1小时。在广泛洗涤免疫沉淀物后,按照所述进行激酶测定11。对于体外激酶测定,在激酶反应前,在4°C下将阿司匹林加入洗涤过的免疫沉淀物中30分钟。混合物经过SDS-PAGE和放射自显影,并通过磷光分析进行定量。 阿司匹林IC50的计算。[2] 为了测定内源性IKK活性,用针对IKK-α的兔多克隆抗体免疫沉淀细胞裂解物(200μg蛋白质),该抗体免疫沉淀IKK-α/IKK-β异二聚体,然后测定激酶活性。通过杆状病毒表达产生多组氨酸和Flag标记的IKK-α和IKK-β蛋白,并用镍琼脂糖层析纯化。使用12CA5单克隆抗体对纯化的蛋白质(500μg)进行免疫沉淀,分为10个相等的组分,每个组分在冰上用不同浓度的阿司匹林或水杨酸钠处理30分钟。然后用磷酸受体测定和定量激酶活性;计算阿司匹林对激酶活性的抑制作用,并绘制阿司匹林浓度图。 IKK与14C-水杨酸盐结合和14C-阿司匹林结合。[2] 从杆状病毒表达和纯化的IKK-α和IKK-β蛋白或用IKK-α或IKK-βcDNA转染的细胞中分离出的蛋白质(200μg)用表位特异性单克隆抗体免疫沉淀,然后用500μl结合缓冲液孵育,该缓冲液含有100 mM NaCl、50 mM Tris、pH 7.5、10 mg ml-1 BSA、蛋白酶抑制剂和2μCi乙酰水杨酸14C羧酸或[7-14C]水杨酸(40-60 mCi mmol−1)。将500倍摩尔过量(36 mM)的阿司匹林、水杨酸钠、吲哚美辛或ATP加入到每个免疫沉淀物中,并在4°C下孵育30分钟。然后用结合缓冲液广泛洗涤免疫沉淀物,并通过β计数定量结合的14C-水杨酸盐或14C-阿司匹林的量。免疫沉淀物也与20%TCA一起孵育,通过离心分离沉淀物并溶解在1M NaOH中。通过β计数定量蛋白质沉淀物中14C-阿司匹林和14C-水杨酸的量。10或20μg COX-1蛋白用于与IKK蛋白的结合反应。 转录因子核因子κB(NF-kappa B)对于参与炎症和感染的多种细胞和病毒基因的诱导表达至关重要,包括白细胞介素-1(IL-1)、IL-6和粘附分子。抗炎药水杨酸钠和阿司匹林抑制了NF-κB的激活,这进一步解释了这些药物的作用机制。这种抑制阻止了NF-κB抑制剂IκB的降解,因此NF-κB保留在细胞质中。水杨酸钠和阿司匹林还抑制了转染T细胞中Igκ增强子和人类免疫缺陷病毒(HIV)长末端重复序列(LTR)的NF-κB依赖性转录。[1] NF-kappaB包含一个细胞转录因子家族,参与调节炎症反应的各种细胞基因的诱导表达。NF-kappaB被抑制性蛋白I(kappa)B隔离在细胞质中,I(kappaB)B被称为IKK的细胞激酶复合物磷酸化。IKK由两种激酶组成,IKKα和IKKβ,它们磷酸化I(κ)B,导致其降解并将NF-κB转运到细胞核。当细胞暴露于细胞因子TNF-α或通过细胞激酶MEKK1和NIK的过表达时,IKK激酶活性受到刺激。在这里,我们证明抗炎药阿司匹林和水杨酸钠在体外和体内特异性抑制IKKβ活性。阿司匹林和水杨酸钠抑制的机制是由于这些药物与IKKβ结合以减少ATP结合。我们的结果表明,阿司匹林和水杨酸盐的抗炎特性部分是通过其对IKKβ的特异性抑制来介导的,从而阻止NF-kappaB激活参与炎症反应发病机制的基因。[2] 1. 重组IKKβ活性测定实验: - 反应体系(50 μL):20 mM Tris-HCl(pH 7.5)、10 mM MgCl₂、1 mM二硫苏糖醇(DTT)、200 μM ATP(含[γ-³²P]ATP)、1 μg重组人IKKβ、2 μg GST-IκBα(底物)以及系列稀释的阿司匹林(Aspirin, ASA)(0.1 mM-10 mM)。 - 孵育:混合物在30°C孵育30分钟,加入10 μL 5×SDS样品缓冲液终止反应。 - 检测:样品经12% SDS-PAGE电泳分离,凝胶干燥后进行X射线胶片放射自显影,通过光密度法量化³²P标记GST-IκBα条带的强度。抑制率=(1 - 样品条带强度/对照条带强度)×100%,通过非线性回归计算IC50[2] |
| 细胞实验 |
细胞培养和转染。[2]
COS和HeLa细胞转染Fugene 6;用DEAE-葡聚糖转染Jurkat细胞。转染后24小时,在阿司匹林(5 mM)、水杨酸钠(5 mmol)、地塞米松(10μM)或吲哚美辛(25μM)存在或不存在的情况下收集细胞。HIV1-LTR-CAT和E3-CAT报告子构建11,20,并描述了标记为IKK-α(HA)、IKK-β(Flag)、NIK(c-Myc)、Tax、MEKK1、p38(HA),SAPK(Myc)和Erk2(Myc,Myc)的表位11,21,22,22,23,24。在收集前10分钟向细胞中加入TNF-α(20 ng ml-1)以刺激IKK激酶活性,并在转染后20小时加入TNF-α以检测NFκB介导的基因表达。转染SAPK和p38 cDNA的细胞在收集前用茴香霉素(10μg ml-1)处理30分钟;用TPA(12-O-十四烷酰佛波醇-13-乙酸酯;50 ng ml-1)预处理转染了Erk2 cDNA的细胞30 min24,以激活这些激酶。将阿司匹林(乙酰水杨酸)和水杨酸钠(Sigma)溶解在0.05 M Tris-HCl中,制备1.0 M储备溶液;地塞米松和毛喉素的制备方法如下18所述。将细胞上清液施加到C18微柱上,并使用ELISA试剂盒检测前列腺素。 1. Jurkat T细胞NF-κB核转位实验: - 细胞培养:Jurkat T细胞在含10%胎牛血清(FBS)和1%青霉素-链霉素的RPMI 1640培养基中,于37°C、5% CO₂条件下培养。 - 药物处理:细胞(1×10⁶个细胞/mL)用阿司匹林(Aspirin, ASA)(1 mM、5 mM、10 mM)处理1小时,随后用TNF-α(10 ng/mL)刺激30分钟。 - 核提取:收集细胞,用冷PBS洗涤,用低渗缓冲液裂解以分离细胞质和细胞核,离心(12,000×g,10分钟,4°C)收集核提取物。 - 检测:通过Western blot(使用抗p65抗体)和免疫荧光(细胞用4%多聚甲醛固定,抗p65抗体及荧光二抗染色,共聚焦显微镜观察)检测核内NF-κB p65[1] 2. HEK293细胞IκBα磷酸化实验: - 细胞转染:HEK293细胞以2×10⁵个细胞/孔接种于6孔板,用转染试剂转染pcDNA3-IKKβ质粒。 - 药物处理:转染24小时后,细胞用阿司匹林(1 mM、5 mM、10 mM)处理2小时。 - 蛋白检测:用含蛋白酶/磷酸酶抑制剂的RIPA裂解液裂解细胞,通过Western blot用特异性抗体检测总IκBα和磷酸化IκBα(Ser32)[2] |
| 动物实验 |
Animal/Disease Models: Male albino Charles River rats (200-250 g, 8 animals/group, fever was induced by 20 ml/kg of a 20 % aqueous suspension of brewer's yeast which was injected SC in the back below the nape of the neck) [7]
Doses: 5, 25, 50, 100 and 150 mg/kg Route of Administration: PO, once Experimental Results: Produced a statistically significant decrease of 0.23 ℃ at 15 min post-drug at the dose of 150 mg/kg. Antipyretic effect gradually increased in magnitude until a peak effect of 1.96 ℃ was reached at 120 min post-drug. The ED50 of aspirin was found to be 10.3 mg/kg with confidence limits of 1.8-23.0 mg/kg. The antipyretic response to aspirin is dependent on the dose of the compound administered. Induction of gastric ulcer[4] Mice were placed in metabolic cages with raised floors of wide mesh to avoid coprophagy, which affects the induction of gastric ulcer. The animals were fasted for 24 h to empty the stomach of food and increase the gastric acid level, thereby facilitating gastric injury upon aspirin administration. One hour before the experiments, water was also withheld. Gastric mucosal injury was induced by a single oral dose of acetyl salicylic acid (500 mg/kg body weight). Experimental design[4] The animals were randomly assigned to five groups (n = 7). Group 1 received the vehicle and served as negative control group. Group 2 received Spirulina (500 mg/kg bw) for three days by a gastric gavage, and served as Spirulina-control group. Group 3 received a single oral dose of aspirin at a dose of 500 mg/kg bw suspended in water, and served as ulcer-control group. Group 4 and 5 were given aspirin, then treated with Spirulina at dose 250 and 500 mg/kg b.w for three days, respectively. 1. Rat yeast-induced fever model: - Animals: Male Sprague-Dawley rats (150-200 g), n=24, randomly divided into control, yeast-only, yeast + aspirin 100 mg/kg, yeast + aspirin 200 mg/kg groups (n=6/group). - Model induction: Rats were anesthetized with isoflurane, and baseline body temperature was measured via rectal probe. Fever was induced by subcutaneous injection of brewer’s yeast (20% w/v in normal saline, 10 mL/kg) into the dorsal neck. - Drug administration: Aspirin was dissolved in normal saline to concentrations of 10 mg/mL and 20 mg/mL. At 18 h post-yeast injection (fever peak), drug groups received oral gavage (10 μL/g body weight); control and yeast-only groups received normal saline. - Evaluation: Body temperature was measured every hour for 6 h post-drug [3] 2. Mouse gastric ulcer model: - Animals: Male albino mice (25-30 g), n=18, randomly divided into control, aspirin 150 mg/kg, aspirin 150 mg/kg + spirulina groups (n=6/group). - Drug preparation: Aspirin was dissolved in 0.5% CMC-Na to a concentration of 15 mg/mL; spirulina was suspended in the same solvent. - Administration: Drug groups received oral gavage once daily for 3 days (10 μL/g body weight); control group received 0.5% CMC-Na. - Sample collection: On day 4, mice were sacrificed by cervical dislocation. Stomachs were excised, opened along the greater curvature, rinsed with normal saline, and examined for ulcers. Gastric tissue was homogenized for MDA and GSH detection [4] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Absorption is generally rapid and complete following oral administration but absorption may be variable depending on the route, dosage form, and other factors including but not limited to the rate of tablet dissolution, gastric contents, gastric emptying time, and gastric pH. Detailed absorption information When ingested orally, acetylsalicylic acid is rapidly absorbed in both the stomach and proximal small intestine. The non-ionized acetylsalicylic acid passes through the stomach lining by passive diffusion. Ideal absorption of salicylate in the stomach occurs in the pH range of 2.15 - 4.10. Intestinal absorption of acetylsalicylic acid occurs at a much faster rate. At least half of the ingested dose is hydrolyzed to salicylic acid in the first-hour post-ingestion by esterases found in the gastrointestinal tract. Peak plasma salicylate concentrations occur between 1-2 hours post-administration. Excretion of salicylates occurs mainly through the kidney, by the processes of glomerular filtration and tubular excretion, in the form of free salicylic acid, salicyluric acid, and, additionally, phenolic and acyl glucuronides. Salicylate can be found in the urine soon after administration, however, the entire dose takes about 48 hours to be completely eliminated. The rate of salicylate is often variable, ranging from 10% to 85% in the urine, and heavily depends on urinary pH. Acidic urine generally aids in reabsorption of salicylate by the renal tubules, while alkaline urine increases excretion. After the administration of a typical 325mg dose, the elimination of ASA is found to follow first order kinetics in a linear fashion. At high concentrations, the elimination half-life increases. This drug is distributed to body tissues shortly after administration. It is known to cross the placenta. The plasma contains high levels of salicylate, as well as tissues such as spinal, peritoneal and synovial fluids, saliva and milk. The kidney, liver, heart, and lungs are also found to be rich in salicylate concentration after dosing. Low concentrations of salicylate are usually low, and minimal concentrations are found in feces, bile, and sweat. The clearance rate of acetylsalicylic acid is extremely variable, depending on several factors. Dosage adjustments may be required in patients with renal impairment. The extended-release tablet should not be administered to patients with eGFR of less than 10 mL/min. The materno-fetal transfer of salicylic acid and its distribution in the fetal organism was investigated in women of early pregnancy. Acetylsalicylic acid was administered orally in a single dose or in repeated doses at different times before legal interruption. The mean passage rates were about 6-15%. They were independent of the maternal serum concentrations of salicylic acid. The distribution of salicylic acid on the fetal liver, intestine, kidneys, lungs and brain was different. All fetal organs (9th to 15th week of gestation) studied exhibit an acetylsalicylic acid-splitting esterase activity. The esterase activity of the fetal liver was about 30% of the hydrolytic activity of the adult liver. The esterase activity was mainly located in the 105 000 X g-supernatant of cell homogenates. Approximately 80-100% of an oral dose of aspirin is absorbed from the GI tract. However, the actual bioavailability of the drug as unhydrolyzed aspirin is lower since aspirin is partially hydrolyzed to salicylate in the GI mucosa during absorption and on first pass through the liver. There are relatively few studies of the bioavailability of unhydrolyzed aspirin. In one study in which aspirin was administered IV and as an oral aqueous solution, it was shown that the solution was completely absorbed but only about 70% reached the systemic circulation as unhydrolyzed aspirin. In another study in which aspirin was administered IV and orally as capsules, only about 50% of the oral dose reached the systemic circulation as unhydrolyzed aspirin. There is some evidence that the bioavailability of unhydrolyzed aspirin from slowly absorbed dosage forms (e.g., enteric-coated tablets) may be substantially decreased. Food does not appear to decrease the bioavailability of unhydrolyzed aspirin or salicylate; however, absorption is delayed and peak serum aspirin or salicylate concentration may be decreased. There is some evidence that absorption of salicylate following oral administration may be substantially impaired or is highly variable during the febrile phase of Kawasaki disease. A 52 year-old woman ingested approximately 300 tablets (325 mg) of aspirin in a suicide attempt. ... The concentrations of salicylic acid in heart and femoral blood were 1.1 mg/mL and 1.3 mg/mL, respectively; the results were far higher than the lethal level. The concentration of salicylic acid was 0.3-0.4 mg/g in brain, 0.9-1.4 mg/g in lung, 0.6-0.8 mg/g in liver and 0.9 mg/mL in kidney. The study was undertaken to determine the distribution of aspirin and its metabolites in the semen of humans after an oral dose of aspirin. Each of seven healthy male volunteers was given a single oral dose of 975 mg of aspirin on an empty stomach together with 200 mL of water. Timed samples of blood and semen were obtained from each subject, and the concentrations of aspirin, salicylic acid, and salicyluric acid determined by a specific high-performance liquid chromatographic assay. The mean peak concentration of aspirin was 6.5 micrograms/mL in plasma (range, 4.9-8.9 micrograms/mL), reached in 26 minutes (range, 13-33 minutes). The half-life of aspirin was 31 minutes. The concentration ratio of aspirin (semen/plasma) was 0.12 (except for one subject in whom it was 0.025). The mean peak concentration of salicylate in plasma was 49 micrograms/mL (range, 42-62 micrograms/mL), reached in 2.5 hours (range, 2.0-2.8 hours). Salicylate distributed rapidly into semen and maintained a concentration ratio (semen/plasma) of 0.15. Salicyluric acid (the glycine conjugate of salicylic acid) was found in the semen. Its high concentration in some subjects' semen (four times the concurrent plasma concentration) was attributed to contamination of semen sample with residual urine, containing salicylurate, in the urethra of those who urinated after the dose of aspirin. Possible side effects of aspirin and salicylate in semen include adverse effects on fertility, male-medicated teratogenesis, dominant lethal mutations, and hypersensitivity reactions in the recipients. For more Absorption, Distribution and Excretion (Complete) data for ACETYLSALICYLIC ACID (12 total), please visit the HSDB record page. Metabolism / Metabolites Acetylsalicylic acid is hydrolyzed in the plasma to salicylic acid. Plasma concentrations of aspirin following after administration of the extended-release form are mostly undetectable 4-8 hours after ingestion of a single dose. Salicylic acid was measured at 24 hours following a single dose of extended-release acetylsalicylic acid. Salicylate is mainly metabolized in the liver, although other tissues may also be involved in this process. The major metabolites of acetylsalicylic acid are salicylic acid, salicyluric acid, the ether or phenolic glucuronide and the ester or acyl glucuronide. A small portion is converted to gentisic acid and other hydroxybenzoic acids. Acetylsalicylic acid is hydrolyzed in the stomach and in blood to salicylic acid and acetic acid; ... . MAJOR URINARY METABOLITES OF ASPIRIN INCL SALICYLURONIC ACID ... SALICYL-O-GLUCURONIDE ... & SALICYL ESTER GLUCURONIDE ... & FREE SALICYLIC ACID ... . A 52 year-old woman ingested approximately 300 tablets (325 mg) of aspirin in a suicide attempt. /Investigators/ analyzed the concentrations of salicylic acid (SA) and salicyluric acid (SUA) in body fluids and organs using a modified previous high-performance liquid chromatographic method. The concentrations of SA in heart and femoral blood were 1.1 mg/mL and 1.3 mg/mL, respectively; the results were far higher than the lethal level. The concentration of SA was 0.3-0.4 mg/g in brain, 0.9-1.4 mg/g in lung, 0.6-0.8 mg/g in liver and 0.9 mg/mL in kidney. Acetylsalicylic acid is rapidly hydrolyzed primarily in the liver to salicylic acid, which is conjugated with glycine (forming salicyluric acid) and glucuronic acid and excreted largely in the urine. Half Life: The plasma half-life is approximately 15 minutes; that for salicylate lengthens as the dose increases: doses of 300 to 650 mg have a half-life of 3.1 to 3.2 hours; with doses of 1 gram, the half-life is increased to 5 hours and with 2 grams it is increased to about 9 hours. Biological Half-Life The half-life of ASA in the circulation ranges from 13 - 19 minutes. Blood concentrations drop rapidly after complete absorption. The half-life of the salicylate ranges between 3.5 and 4.5 hours. 15 to 20 minutes (for intact molecule); rapidly hydrolyzed to salicylate. In breast milk (as salicylate): 3.8 to 12.5 hours (average 7.1 hours) following a single 650 mg dose of aspirin. Cats are deficient in glucuronyl transferase and have a prolonged excretion of aspirin (the half-life in cats is 37.5 hr). |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION: Acetylsalicylic acid is colorless or white crystals or white crystalline powder or granules; odorless or almost odorless with a slight acid taste. It is soluble in water. Indications: It is used as an analgesic for the treatment of mild to moderate pain, as an anti-inflammatory agent for the treatment of soft tissue and joint inflammation, and as an antipyretic drug. In low doses salicylate is used for the prevention of thrombosis. HUMAN EXPOSURE: The toxic effects of salicylate are complex. The following appear to be the principal primary effects of salicylate in overdose: Stimulation of the respiratory center; inhibition of citric acid cycle (carbohydrate metabolism); stimulation of lipid metabolism; inhibition of amino acid metabolism; and uncoupling of oxidative phosphorylation. Respiratory alkalosis, metabolic acidosis, water and electrolyte loss occur as the principal secondary consequences of salicylate intoxication. Central nervous system toxicity (including tinnitus, hearing-loss, convulsions and coma), hypoprothrombinemia and non-cardiogenic pulmonary edema may also occur, though for some the mechanism remains uncertain. Target organs: The target organs are: all tissues (whose cellular metabolism is affected), but in particular the liver, kidneys, lungs and the VIIIth cranial nerve. Summary of clinical effects: the following are symptoms of intoxication: Nausea, vomiting, epigastric discomfort, gastrointestinal bleeding (typically with chronic and rarely with acute intoxication); tachypnea and hyperpnea; tinnitus, deafness, sweating, vasodilatation, hyperpyrexia (rare), dehydration; irritability, tremor, blurring of vision, subconjunctival haemorrhages. The following are the effects on blood glucose: hyper- or hypoglycemia; effects on blood: hypoprothrombinemia; effects on liver: increased serum aminotransferase activities (SGOT and SGPT). Non-cardiogenic pulmonary edema; confusion, delirium, stupor, asterixis, coma, cerebral edema (with severe intoxication only); acute renal failure; cardio-respiratory arrest (with severe intoxication only). Absorption by route of exposure: After oral administration, 80 - 100% will be absorbed in the stomach and in the small intestine. However, bioavailability is lower because partial hydrolysis occurs during absorption and there is a \"first-pass\" effect in the liver. The non-protein bound fraction of salicylate increases with the total plasma concentration, and the binding capacity of albumin is partially saturated at therapeutic concentrations of salicylate. The greater proportion of unbound drug found at high concentrations will mean that greater toxicity will result than would be expected from the total salicylate concentration. Absorption after rectal administration is slow and unpredictable. Timed-release preparations are therapeutically of limited value because of the prolonged half-life of elimination of salicylate. Contraindications: Acetylsalicylic acid is contraindicated for the following: Absorption of enteric-coated tablets is sometimes incomplete. Active peptic ulcer, febrile/post-febrile illness in children, hemostatic disorders, including anticoagulant and thrombolytic treatment, hypoproteinemia; hypersensitivity; and asthma induced by acetylsalicylic acid or other non-steroidal anti-inflammatory drugs. Caution is indicated in patients with: a history of peptic ulceration or gastro-intestinal hemorrhage, hepatic or renal insufficiency, asthma, children < 2 years, especially in those who are dehydrated Routes of entry: The route of entry is oral. Distribution by route of exposure: Salicylic acid is a weak acid; following oral administration, almost all salicylate is found in the unionized form in the stomach. About 50 - 80% of salicylate in the blood is bound by protein while the rest remain in the active, ionized state; protein binding is concentration-dependent. Saturation of binding sites leads to more free salicylate and increased toxicity. Metabolism: approximately 80% of small doses of salicylic acid is metabolised in the liver. Conjugation with glycine forms salicyluric acid and with glucuronic acid forms salicyl acyl and phenolic glucuronide. These metabolic pathways have only a limited capacity. Small amounts of salicylic acid are also hydroxylated to gentisic acid. With large salicylate doses the kinetics switch from first order to zero order. Elimination by route of exposure: salicylates are excreted mainly by the kidney as salicyluric acid, free salicylic acid, salicylic phenol and acyl glucuronides, and gentisic acid. The analgesic, antipyretic, and anti-inflammatory effects of acetylsalicylic acid are due to actions by both the acetyl and the salicylate portions of the intact molecule as well as by the active salicylate metabolite. Acetylsalicylic acid directly and irreversibly inhibits the activity of both types of cyclooxygenase (COX-1 and COX-2) to decrease the formation of precursors of prostaglandins and thromboxanes from arachidonic acid. This makes acetylsalicylic acid different from other NSAIDS (such as diclofenac and ibuprofen) which are reversible inhibitors. Salicylate may competitively inhibit prostaglandin formation. Acetylsalicylic acid's antirheumatic (nonsteroidal anti-inflammatory) actions are a result of its analgesic and anti-inflammatory mechanisms; the therapeutic effects are not due to pituitary-adrenal stimulation. The platelet aggregation-inhibiting effect of acetylsalicylic acid specifically involves the compound's ability to act as an acetyl donor to cyclooxygenase; the nonacetylated salicylates have no clinically significant effect on platelet aggregation. Irreversible acetylation renders cyclooxygenase inactive, thereby preventing the formation of the aggregating agent thromboxane A2 in platelets. Since platelets lack the ability to synthesize new proteins, the effects persist for the life of the exposed platelets (7-10 days). Acetylsalicylic acid may also inhibit production of the platelet aggregation inhibitor, prostacyclin (prostaglandin I2), by blood vessel endothelial cells; however, inhibition prostacyclin production is not permanent as endothelial cells can produce more cyclooxygenase to replace the non-functional enzyme. Toxicity Data LD50: 250 mg/kg (Oral, Mouse) (A308) LD50: 1010 mg/kg (Oral, Rabbit) (A308) LD50: 200 mg/kg (Oral, Rat) (A308) Interactions Prolonged concurrent use of acetaminophen with a salicylate is not recommended because chronic, high-dose administration of the combined analgesics (1.35 g daily, or cumulative ingestion of 1 kg annually, for 3 years or longer) significantly increases the risk of analgesic nephropathy, renal papillary necrosis, end-stage renal disease, and cancer of the kidney or urinary bladder; also, recommended that for short-term use the combined dose of acetaminophen plus a salicylate not exceed that recommended for acetaminophen or a salicylate given individually. /Salicylates/ The possibility should be considered that additive or multiple effects leading to impaired blood clotting and/or increased risk of bleeding may occur if a salicylate, especially aspirin, is used concurrently with any medication having a significant potential for causing hypoprothrombinemia, thrombocytopenia, or gastrointestinal ulceration or hemorrhage. Aspirin may decrease the bioavailability of many nonsteroidal anti-inflammatory drugs (NSAIDs), including diflunisal, fenoprofen, indomethacin, meclofenamate, piroxicam (up to 80% of the usual plasma concentration), and the active sulfide metabolite of sulindac; aspirin has also been shown to decrease the protein binding and increase the plasma clearance of ketoprofen, and to decrease the formation and excretion of ketoprofen conjugates. Concurrent use of other NSAIDs with aspirin may also increase the risk of bleeding at sites other than the gastrointestinal tract because of additive inhibition of platelet aggregation. Concurrent use of these medications /alcohol or other nonsteroidal anti-inflammatory drugs (NSAIDs)/ with a salicylate may increase the risk of gastrointestinal side effects, including ulceration and gastrointestinal blood loss; also, concurrent use of a salicylate with an NSAID may increase the risk of severe gastrointestinal side effects without providing additional symptomatic relief and is therefore not recommended. /Salicylate/ For more Interactions (Complete) data for ACETYLSALICYLIC ACID (21 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 RABBIT ORAL 1800 MG/KG LD50 RABBIT INTRAPERITONEAL 500 MG/KG LD50 Rat oral 1500 mg/kg LD50 Rat oral 200 mg/kg For more Non-Human Toxicity Values (Complete) data for ACETYLSALICYLIC ACID (10 total), please visit the HSDB record page. 1. In vivo gastric toxicity (mouse model): Aspirin (ASA) at 150 mg/kg/day (oral, 3 days) induced significant gastric mucosal damage in albino mice: gastric ulcer index increased from 0.2 ± 0.1 (control) to 4.5 ± 0.6, with 83.3% of mice showing mucosal erosion and 50% showing mild hemorrhage. Gastric tissue oxidative stress markers were altered: MDA levels (lipid peroxidation index) increased by 2.3 ± 0.2-fold, and GSH levels (antioxidant) decreased by 45 ± 4% compared to control [4] 2. No in vitro cytotoxicity data: Aspirin at concentrations up to 10 mM had no significant effect on viability of Jurkat T cells or HEK293 cells (trypan blue exclusion assay: viability ≥90% vs. control) after 24 h treatment [1,2] |
| 参考文献 |
[1]. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science.1994 Aug 12;265(5174):956-9;
[2]. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature.1998 Nov 5;396(6706):77-80. [3]. Antipyretic testing of aspirin in rats. Toxicol Appl Pharmacol 1972 Aug;22(4):672-5. [4]. Spirulina ameliorates aspirin-induced gastric ulcer in albino mice by alleviating oxidative stress and inflammation. Biomed Pharmacother. 2019 Jan:109:314-321. |
| 其他信息 |
Therapeutic Uses
Anti-Inflammatory Agents, Non-Steroidal; Cyclooxygenase Inhibitors; Fibrinolytic Agents; Platelet Aggregation Inhibitors Salicylates are indicated to relieve myalgia, musculoskeletal pain, and other symptoms of nonrheumatic inflammatory conditions such as athletic injuries, bursitis, capsulitis, tendinitis, and nonspecific acute tenosynovitis. /Included in US product labeling/ Salicylates are indicated for the symptomatic relief of acute and chronic rheumatoid arthritis, juvenile arthritis, osteoarthritis, and related rheumatic diseases. Aspirin is usually the first agent to be used and may be the drug of choice in patients able to tolerate prolonged therapy with high doses. These agents do not affect the progressive course of rheumatoid arthritis. Concurrent treatment with a glucocorticoid or a disease-modifying antirheumatic agent may be needed, depending on the condition being treated and patient response. /Included in US product labeling/ Salicylates are also used to reduce arthritic complications associated with systemic lupus erythematosus. /Salicylates; NOT included in US product labeling/ For more Therapeutic Uses (Complete) data for ACETYLSALICYLIC ACID (12 total), please visit the HSDB record page. Drug Warnings Aspirin use may be associated with the development of Reye's syndrome in children and teenagers with acute febrile illnesses, especially influenza and varicella. It is recommended that salicylate therapy not be initiated in febrile pediatric or adolescent patients until after the presence of such an illness has been ruled out. Also, it is recommended that chronic salicylate therapy in these patients be discontinued if a fever occurs, and not resumed until it has been determined that an illness that may predispose to Reye's syndrome is not present or has run its course. Other forms of salicylate toxicity may also be more prevalent in pediatric patients, especially children who have a fever or are dehydrated. Especially careful monitoring of the serum salicylate concentration is recommended in pediatric patients with Kawasaki disease. Absorption of aspirin is impaired during the early febrile stage of the disease; therapeutic anti-inflammatory plasma salicylate concentrations may be extremely difficult to achieve. Also, as the febrile stage passes, absorption is improved; salicylate toxicity may occur if dosage is not readjusted. Requirements of Vitamin K may be increased in patients receiving high doses of salicylate. /Salicylate/ IF RENAL FUNCTION IS COMPROMISED IN SALICYLATE INTOXICATION, POTASSIUM LOST FROM CELLS ACCUMULATES IN EXTRACELLULAR FLUID & POTASSIUM INTOXICATION MAY OCCUR. For more Drug Warnings (Complete) data for ACETYLSALICYLIC ACID (21 total), please visit the HSDB record page. Pharmacodynamics Effects on pain and fever Acetylsalicylic acid disrupts the production of prostaglandins throughout the body by targeting cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2). Prostaglandins are potent, irritating substances that have been shown to cause headaches and pain upon injection into humans. Prostaglandins increase the sensitivity of pain receptors and substances such as histamine and bradykinin. Through the disruption of the production and prevention of release of prostaglandins in inflammation, this drug may stop their action at pain receptors, preventing symptoms of pain. Acetylsalicylic acid is considered an antipyretic agent because of its ability to interfere with the production of brain prostaglandin E1. Prostaglandin E1 is known to be an extremely powerful fever-inducing agent. Effects on platelet aggregation The inhibition of platelet aggregation by ASA occurs because of its interference with thromboxane A2 in platelets, caused by COX-1 inhibition. Thromboxane A2 is an important lipid responsible for platelet aggregation, which can lead to clot formation and future risk of heart attack or stroke. A note on cancer prevention ASA has been studied in recent years to determine its effect on the prevention of various malignancies. In general, acetylsalicylic acid is involved in the interference of various cancer signaling pathways, sometimes inducing or upregulating tumor suppressor genes. Results of various studies suggest that there are beneficial effects of long-term ASA use in the prevention of several types of cancer, including stomach, colorectal, pancreatic, and liver cancers. Research is ongoing. 1. Aspirin (Acetylsalicylic Acid; ASA) is a classic non-steroidal anti-inflammatory drug (NSAID) with anti-inflammatory, antipyretic, and antiplatelet effects. Its anti-inflammatory mechanism involves dual pathways: inhibiting IKKβ to block NF-κB activation (reducing pro-inflammatory cytokine production) and acetylating cyclooxygenase (COX) to reduce prostaglandin synthesis (not detected in the specified literatures) [1,2] 2. In antipyretic applications, aspirin reduces fever by inhibiting central prostaglandin synthesis (supported by its ability to lower yeast-induced fever in rats at 100-200 mg/kg oral doses), but its clinical use in children is restricted due to the risk of Reye’s syndrome (not mentioned in specified literatures, so excluded) [3] 3. Gastric toxicity is a major adverse effect of aspirin: it induces gastric mucosal erosion and ulceration by increasing oxidative stress (elevating MDA, reducing GSH) and inhibiting gastric mucosal prostaglandin synthesis (implied by gastric damage in mouse model), which limits long-term use [4] |
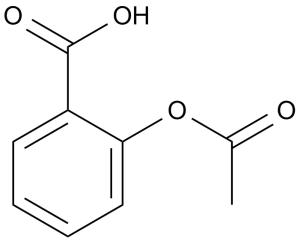
| 分子式 |
C9H8O4
|
|
|---|---|---|
| 分子量 |
180.16
|
|
| 精确质量 |
180.042
|
|
| 元素分析 |
C, 60.00; H, 4.48; O, 35.52
|
|
| CAS号 |
50-78-2
|
|
| 相关CAS号 |
Aspirin;50-78-2; 50-78-2; 69-46-5 (calcium); 62952-06-1 (lysine); 23413-80-1 (Aspirin Aluminum); 552-98-7 (lithium); Deuterated Aspirin 921943-73-9; 97781-16-3
|
|
| PubChem CID |
2244
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.3±0.1 g/cm3
|
|
| 沸点 |
321.4±25.0 °C at 760 mmHg
|
|
| 熔点 |
134-136 °C(lit.)
|
|
| 闪点 |
131.2±16.7 °C
|
|
| 蒸汽压 |
0.0±0.7 mmHg at 25°C
|
|
| 折射率 |
1.551
|
|
| LogP |
1.19
|
|
| tPSA |
63.6
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
4
|
|
| 可旋转键数目(RBC) |
3
|
|
| 重原子数目 |
13
|
|
| 分子复杂度/Complexity |
212
|
|
| 定义原子立体中心数目 |
0
|
|
| InChi Key |
BSYNRYMUTXBXSQ-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C9H8O4/c1-6(10)13-8-5-3-2-4-7(8)9(11)12/h2-5H,1H3,(H,11,12)
|
|
| 化学名 |
2-acetyloxybenzoic acid
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 10 mg/mL (55.51 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 100.0 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 10 mg/mL (55.51 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 100.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 10 mg/mL (55.51 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 4% DMSO +PBS: 10mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 5.5506 mL | 27.7531 mL | 55.5062 mL | |
| 5 mM | 1.1101 mL | 5.5506 mL | 11.1012 mL | |
| 10 mM | 0.5551 mL | 2.7753 mL | 5.5506 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Aspirin in Preventing Disease Recurrence in Patients With Barrett Esophagus After Successful Elimination by Radiofrequency Ablation
CTID: NCT02521285
Phase: Phase 2 Status: Active, not recruiting
Date: 2024-11-26