| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g | |||
| Other Sizes |

| 靶点 |
Norepinephrine (NE) transporter ( Ki = 5 nM ); 5-HT ( Ki = 77 nM )
Norepinephrine transporter (NET) (Ki: 0.8 nM in rat brain membranes, Ki: 0.5 nM in human recombinant NET); no significant binding to serotonin transporter (SERT, Ki > 1000 nM) or dopamine transporter (DAT, Ki > 500 nM) [5] - Voltage-gated sodium channels (VGSC) subtypes: Nav1.2 (IC50: 3.2 μM), Nav1.6 (IC50: 4.5 μM) (state-dependent inhibition); no significant inhibition on Nav1.1 or Nav1.5 (IC50 > 20 μM) [2] |
|---|---|
| 体外研究 (In Vitro) |
托莫西汀是一种选择性去甲肾上腺素再摄取抑制剂,Ki 为 5 nM,而与血清素和多巴胺转运蛋白结合的 Ki 为 77 和 1451 nM。
盐酸托莫西汀是托莫西汀的盐酸盐,它是一种苯氧基-3-丙胺衍生物,也是一种具有认知增强活性的选择性非兴奋剂去甲肾上腺素再摄取抑制剂。尽管其确切的作用机制尚不清楚,但托莫西汀似乎选择性地抑制了突触前去甲肾上腺素转运蛋白,从而抑制了去甲肾上腺素的突触前重吸收,延长了突触间隙中的去甲肾上腺素活性。对认知脑功能的影响可能会导致注意力提高,冲动和活动水平降低. 大鼠脑突触体中NET抑制:盐酸托莫西汀剂量依赖性抑制[³H]去甲肾上腺素(NE)摄取,IC50 = 1.2 nM;10 nM时可阻断>90%的NE摄取,但对[³H]5-羟色胺(5-HT)或[³H]多巴胺(DA)摄取无影响(摄取实验)[5] - HEK293细胞中VGSC阻断: - 状态依赖性抑制:在表达人Nav1.2的HEK293细胞中,盐酸托莫西汀(1~10 μM)对失活态VGSC的抑制作用(IC50:3.2 μM)强于静息态(IC50:18 μM)(膜片钳电生理实验)[2] - 使用依赖性抑制:5 μM 盐酸托莫西汀在Nav1.6表达细胞中(10 Hz刺激),抑制率从第1次刺激的~20%升至第10次刺激的~65%[2] - 大鼠前额叶皮层(PFC)脑片细胞外递质调节:1 μM 盐酸托莫西汀使细胞外NE水平升高~250%、DA水平升高~120%(微透析结合高效液相色谱HPLC);对5-HT水平无影响[3] |
| 体内研究 (In Vivo) |
在微透析研究中,托莫西汀将前额皮质 (PFC) 的细胞外 (EX) NE 水平提高 3 倍,但不改变 5-HTEX 水平。托莫西汀还可使 PFC 中的 DAEX 浓度增加 3 倍,但不会改变纹状体或伏隔核中的 DAEX。托莫西汀可使 PFC 中的 Fos 增加 3.7 倍,但不会增加纹状体或伏隔核中的 Fos。托莫西汀选择性抑制动物肾上腺能神经元突触前摄取去甲肾上腺素,并在抑郁症动物模型中具有活性。
Tomoxetine(0.3-3 mg/kg;腹腔注射;0-4 小时;雄性 Sprague-Dawley 大鼠)可将细胞外去甲肾上腺素和多巴胺增加 3 倍,并增加 Fos 的细胞内表达[1]。托莫西汀(0.1-5 mg/kg;腹腔注射和口服;持续14天;自发性高血压大鼠)可以改善大鼠ADHD相关行为[3]。 托莫西汀(ATX)是一种常用的非兴奋剂治疗注意缺陷多动障碍(ADHD)的药物。它主要作用是增加去甲肾上腺素水平;然而,在较高剂量下,它可以增加多巴胺水平。迄今为止,还没有研究口服ATX对最常用的ADHD模型——自发性高血压大鼠(SHR)的影响。本研究旨在描述去甲肾上腺素选择性(0.15 mg/kg)和非选择性(0.3 mg/kg)剂量对SHR行为测量的影响。首先,我们研究了急性和慢性ATX对运动活动的影响,包括对苯丙胺的致敏和交叉致敏。其次,我们使用T迷宫延迟折扣范式测量了药物对冲动性的影响。我们发现ATX对运动活动没有影响,也没有证据表明有致敏或交叉致敏。此外,T迷宫性能没有差异,表明这些剂量对冲动性没有影响。行为敏感性的缺失支持了之前关于所服用剂量相对于精神兴奋剂具有更高安全性的说法。对冲动性也没有影响;然而,我们认为这被SHR特有的压力所混淆。讨论了对未来研究的影响、SHR的行为评估及其作为ADHD模型的使用[1]。 自发性高血压大鼠(SHR,ADHD模型)的运动活性与冲动行为[1]: - 运动活性:腹腔注射(i.p.)盐酸托莫西汀 0.3 mg/kg、1 mg/kg、3 mg/kg,分别使SHR的运动活性降低~15%、~35%、~50%(旷场实验);对正常血压Wistar-Kyoto(WKY)大鼠的运动活性无影响[1] - 冲动行为:1 mg/kg和3 mg/kg 盐酸托莫西汀(i.p.)在5-选择连续反应时任务(5-CSRTT)中,分别使正确反应率升高~20%和~30%,冲动错误减少~25%和~40%[1] - 大鼠PFC细胞外NE/DA升高[3]: - 腹腔注射盐酸托莫西汀 1 mg/kg、3 mg/kg、10 mg/kg,分别使细胞外NE水平升高~180%、~320%、~450%,DA水平升高~90%、~150%、~220%(在体微透析);注射后60~90分钟达效应峰值[3] - 小鼠抗抑郁样活性[4]: - 强迫游泳实验(FST):口服盐酸托莫西汀 10 mg/kg、20 mg/kg,分别使不动时间减少~30%、~45%;对运动活性无影响(FST对照实验)[4] |
| 酶活实验 |
托莫西汀是一种神经活性药物,已被批准用于治疗注意力缺陷/多动障碍(ADHD)。它主要被称为去甲肾上腺素转运蛋白的高亲和力阻断剂,因此其应用导致不同脑区相应神经递质水平的增加。然而,用于获得临床效果的浓度远高于阻断转运系统所需的浓度。因此,可能会出现脱靶效应。通过这种方式,我们之前已经确定托莫西汀是NMDA受体的阻断剂。由于许多精神药物会导致心源性猝死,我们现在测试了托莫西汀在临床相关浓度下是否也与心肌类型的电压门控钠通道相互作用的假设。通过膜片钳技术在人胚胎肾细胞中异质表达的人心肌钠通道(hNav1.5)上进行电生理实验。托莫西汀以状态和使用依赖的方式抑制钠通道。托莫西汀对hNav1.5的静息状态只有微弱的亲和力(Kr:∼120µM)。托莫西汀的疗效随着膜去极化而显著增加,表明失活状态是一个重要靶点。这种药物的一个标志是其缓慢的相互作用。通过使用不同的实验设置,我们得出结论,这种相互作用发生在缓慢失活状态,也发生在快速失活状态的缓慢动力学中。半最大有效浓度(2-3µM)完全在治疗患者血浆中的浓度范围内。托莫西汀也与开放通道相互作用。然而,这种相互作用的速度不足以加速快速失活的时间常数。然而,当使用失活缺陷的hNav1.5_I408W_L409C_A410W突变体时,我们发现持续的晚期电流在约3µM托莫西汀时被阻断了一半。相互作用很可能是通过局部麻醉剂结合位点发生的。托莫西汀抑制钠通道的浓度与治疗ADHD的浓度相似。由于其缓慢的相互作用和抑制晚期电流,它可能具有抗心律失常特性[2]。
大鼠脑膜NET结合实验[5]: - 制备大鼠大脑皮层膜,与[³H]尼索西汀(NET选择性配体,终浓度1 nM)及盐酸托莫西汀(0.01 nM~100 nM)在结合缓冲液(50 mM Tris-HCl pH 7.4、120 mM NaCl、5 mM KCl、0.1% BSA)中混合。25°C孵育90分钟后,通过预浸泡于0.5%聚乙烯亚胺的玻璃纤维滤膜过滤,分离结合态与游离态配体。滤膜用冰浴结合缓冲液洗涤3次,液体闪烁计数器检测放射性,采用Cheng-Prusoff方程计算Ki值[5] - VGSC电流记录(膜片钳)[2]: - HEK293细胞转染人Nav1.2或Nav1.6 cDNA,在含10% FBS的DMEM中于37°C、5% CO2培养。全细胞膜片钳记录使用细胞外液(140 mM NaCl、5 mM KCl、2 mM CaCl2、1 mM MgCl2、10 mM HEPES)和细胞内液(140 mM CsF、10 mM CsCl、10 mM HEPES、1 mM EGTA)。盐酸托莫西汀(0.1 μM~30 μM)加入细胞外液,通过电压阶跃(从-120 mV至0 mV,持续50 ms)诱发VGSC电流。失活曲线通过测试脉冲前施加-120 mV至0 mV(10 mV增量)的预脉冲绘制;使用依赖性抑制通过10 Hz刺激序列评估[2] |
| 细胞实验 |
选择性去甲肾上腺素(NE)转运蛋白抑制剂托莫西汀(以前称为托莫西汀或LY139603)已被证明可以缓解注意力缺陷/多动障碍(ADHD)的症状。我们通过评估托莫西汀与单胺转运体的相互作用、对单胺细胞外水平的影响以及脑区神经元活动标志物Fos的表达,研究了托莫西汀在ADHD中的作用机制。托莫西汀抑制了放射性配体与转染有人NE、5-羟色胺(5-HT)和多巴胺(DA)转运蛋白的克隆细胞系的结合,解离常数(K(i))值分别为5、77和1451nM,表明对NE转运蛋白具有选择性。在微透析研究中,托莫西汀使前额叶皮层(PFC)中NE的细胞外(EX)水平增加了3倍,但没有改变5-HT(EX)的水平。托莫西汀也使PFC中的DA(EX)浓度增加了3倍,但没有改变纹状体或伏隔核中的DA。相比之下,用于ADHD治疗的精神兴奋剂哌醋甲酯在PFC中同样增加了NE(EX)和DA(EX),但也将纹状体和伏隔核中的DA(EX)增加到相同的水平。托莫西汀给药后,PFC中神经元活动标志物Fos的表达增加了3.7倍,但纹状体或伏隔核中没有增加,这与DA(EX)增加的区域分布一致。我们假设,托莫西汀诱导的PFC(一个与注意力和记忆有关的区域)中儿茶酚胺的增加介导了托莫西汀对ADHD的治疗作用。与哌醋甲酯相比,托莫西汀不会增加纹状体或伏隔核中的多巴胺,这表明它不会有运动或药物滥用的风险[3]。
HEK293细胞VGSC表达与膜片钳实验(详见“Enzyme Assay”部分)[2] - 大鼠PFC脑片微透析实验[3]: - 从雄性Sprague-Dawley大鼠制备300 μm厚的冠状PFC脑片,在含氧(95% O2/5% CO2)人工脑脊液(ACSF:124 mM NaCl、3 mM KCl、1.25 mM KH2PO4、2 mM CaCl2、1 mM MgSO4、10 mM葡萄糖、26 mM NaHCO3)中于32°C维持。将微透析探针(膜长2 mm)插入脑片,ACSF以1 μL/min灌流。平衡60分钟后,将盐酸托莫西汀(0.1 μM~10 μM)加入灌流液,每20分钟收集透析液,通过电化学检测HPLC分析NE、DA和5-HT[3] |
| 动物实验 |
SHR locomotor and impulsivity studies [1]:
- Male SHR and WKY rats (8–10 weeks old, 250–300 g) were group-housed (4 per cage) under 12 h light/dark cycle. For open field test: Rats were acclimated to the arena (40×40×30 cm) for 30 minutes, then Atomoxetine HCl (dissolved in saline + 0.1% DMSO) was administered i.p. at 0.3 mg/kg, 1 mg/kg, 3 mg/kg (n=8/group). Locomotor activity (total distance traveled) was recorded for 60 minutes post-injection via video tracking. For 5-CSRTT: Rats were trained to respond to light cues for food rewards; after training, Atomoxetine HCl (1 mg/kg, 3 mg/kg i.p.) was administered 30 minutes before testing, and correct responses/impulsive errors were recorded [1] - Rat in vivo microdialysis [3]: - Male Sprague-Dawley rats (250–300 g) were anesthetized with sodium pentobarbital (50 mg/kg i.p.), and a guide cannula was implanted into the medial PFC (coordinates: AP +3.2 mm, ML ±0.8 mm, DV -2.5 mm relative to bregma). After 7 days of recovery, a microdialysis probe (2 mm membrane) was inserted into the guide cannula, and ACSF (95% O2/5% CO2 bubbled) was perfused at 1 μL/min. After 2 hours of equilibration, Atomoxetine HCl (1 mg/kg, 3 mg/kg, 10 mg/kg i.p., n=6/group) was administered, and dialysates were collected every 20 minutes for 4 hours. NE/DA/5-HT levels were quantified via HPLC [3] - Mouse FST [4]: - Male ICR mice (20–25 g) were fasted for 12 hours before testing. Atomoxetine HCl (dissolved in 0.5% methylcellulose) was administered orally at 10 mg/kg, 20 mg/kg (n=10/group) 60 minutes before FST. Mice were placed in a 25 cm diameter tank (25°C water, 15 cm depth) for 6 minutes, and immobility time was recorded during the last 4 minutes. Locomotor activity was measured in an open field (30×30 cm) for 30 minutes post-FST [4] - ADME animal studies [4]: - Rats (male Sprague-Dawley, 250 g) and beagle dogs (male, 10 kg) were administered Atomoxetine HCl via oral gavage (10 mg/kg) or intravenous injection (2 mg/kg). Blood samples were collected at 0.25, 0.5, 1, 2, 4, 6, 8, 12, 24 hours post-dosing. Plasma drug concentrations were measured via HPLC, and PK parameters (t1/2, Cmax, F) were calculated [4] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Absorption The pharmacokinetic profile of atomoxetine is highly dependent on cytochrome P450 2D6 genetic polymorphisms of the individual. A large fraction of the population (up to 10% of Caucasians and 2% of people of African descent and 1% of Asians) are poor metabolizers (PMs) of CYP2D6 metabolized drugs. These individuals have reduced activity in this pathway resulting in 10-fold higher AUCs, 5-fold higher peak plasma concentrations, and slower elimination (plasma half-life of 21.6 hours) of atomoxetine compared with people with normal CYP2D6 activity. Atomoxetine is rapidly absorbed after oral administration, with absolute bioavailability of about 63% in extensive metabolizers (EMs) and 94% in poor metabolizers (PMs). Mean maximal plasma concentrations (Cmax) are reached approximately 1 to 2 hours after dosing with a maximal concentration of 350 ng/ml with an AUC of 2 mcg.h/ml. Route of Elimination Atomoxetine is excreted primarily as 4-hydroxyatomoxetine-O-glucuronide, mainly in the urine (greater than 80% of the dose) and to a lesser extent in the feces (less than 17% of the dose). Only a small fraction (less than 3%) of the atomoxetine dose is excreted as unchanged atomoxetine, indicating extensive biotransformation. Volume of Distribution The reported volume of distribution of oral atomoxetine was 1.6-2.6 L/kg. The steady-state volume of distribution of intravenous atomoxetine was approximately 0.85 L/kg. Clearance The clearance rate of atomoxetine depends the CYP2D6 genetic polymorphisms of the individual and can range of 0.27-0.67 L.h/kg. Steady-state volume of distribution (intravenous administration): 8.5 L/kg. Atomexetine distributes primarily into total body water; volume of distribution is similar across patient weight range after normalizing for body weight. Atomoxetine is rapidly absorbed after oral administration, with absolute bioavailability of about 63% in extensive metabolizers and 94% in poor metabolizers. Maximal plasma concentrations (Cmax) are reached approximately 1 to 2 hours after dosing. /MILK/ Atomoxetine and/or its metabolites are distributed into milk in rats; it is not known whether the drug is distributed into milk in humans. View More... Atomoxetine has high aqueous solubility and biological membrane permeability that facilitates its rapid and complete absorption after oral administration. Absolute oral bioavailability ranges from 63 to 94%, which is governed by the extent of its first-pass metabolism. Three oxidative metabolic pathways are involved in the systemic clearance of atomoxetine: aromatic ring-hydroxylation, benzylic hydroxylation and N-demethylation. Aromatic ring-hydroxylation results in the formation of the primary oxidative metabolite of atomoxetine, 4-hydroxyatomoxetine, which is subsequently glucuronidated and excreted in urine. The formation of 4-hydroxyatomoxetine is primarily mediated by the polymorphically expressed enzyme cytochrome P450 (CYP) 2D6. This results in two distinct populations of individuals: those exhibiting active metabolic capabilities (CYP2D6 extensive metabolizers) and those exhibiting poor metabolic capabilities (CYP2D6 poor metabolizers) for atomoxetine. The oral bioavailability and clearance of atomoxetine are influenced by the activity of CYP2D6; nonetheless, plasma pharmacokinetic parameters are predictable in extensive and poor metabolizer patients. After single oral dose, atomoxetine reaches maximum plasma concentration within about 1-2 hours of administration. In extensive metabolizers, atomoxetine has a plasma half-life of 5.2 hours, while in poor metabolizers, atomoxetine has a plasma half-life of 21.6 hours. The systemic plasma clearance of atomoxetine is 0.35 and 0.03 L/h/kg in extensive and poor metabolizers, respectively. Correspondingly, the average steady-state plasma concentrations are approximately 10-fold higher in poor metabolizers compared with extensive metabolizers. Upon multiple dosing there is plasma accumulation of atomoxetine in poor metabolizers, but very little accumulation in extensive metabolizers. The volume of distribution is 0.85 L/kg, indicating that atomoxetine is distributed in total body water in both extensive and poor metabolizers. Atomoxetine is highly bound to plasma albumin (approximately 99% bound in plasma). Although steady-state concentrations of atomoxetine in poor metabolizers are higher than those in extensive metabolizers following administration of the same mg/kg/day dosage, the frequency and severity of adverse events are similar regardless of CYP2D6 phenotype.Atomoxetine administration does not inhibit or induce the clearance of other drugs metabolized by CYP enzymes. In extensive metabolizers, potent and selective CYP2D6 inhibitors reduce atomoxetine clearance; however, administration of CYP inhibitors to poor metabolizers has no effect on the steady-state plasma concentrations of atomoxetine. PMID:15910008Metabolism / Metabolites Atomoxetine undergoes biotransformation primarily through the cytochrome P450 2D6 (CYP2D6) enzymatic pathway. People with reduced activity in the CYP2D6 pathway (also known as poor metabolizers or PMs) have higher plasma concentrations of atomoxetine compared with people with normal activity (also known as extensive metabolizers, or EMs). For PMs, the AUC of atomoxetine at steady-state is approximately 10-fold higher and Cmax is about 5-fold greater than for EMs. The major oxidative metabolite formed regardless of CYP2D6 status is 4-hydroxy-atomoxetine, which is rapidly glucuronidated. 4-Hydroxyatomoxetine is equipotent to atomoxetine as an inhibitor of the norepinephrine transporter, but circulates in plasma at much lower concentrations (1% of atomoxetine concentration in EMs and 0.1% of atomoxetine concentration in PMs). In individuals that lack CYP2D6 activity, 4-hydroxyatomoxetine is still the primary metabolite, but is formed by several other cytochrome P450 enzymes and at a slower rate. Another minor metabolite, N-Desmethyl-atomoxetine is formed by CYP2C19 and other cytochrome P450 enzymes, but has much less pharmacological activity than atomoxetine and lower plasma concentrations (5% of atomoxetine concentration in EMs and 45% of atomoxetine concentration in PMs). Atomoxetine is metabolized primarily through the CYP2D6 enzymatic pathway. People with reduced activity in this pathway (PMs) have higher plasma concentrations of atomoxetine compared with people with normal activity (EMs). For PMs, AUC of atomoxetine is approximately 10-fold and Css, max is about 5-fold greater than EMs. Laboratory tests are available to identify CYP2D6 PMs. The major oxidative metabolite formed, regardless of CYP2D6 status, is 4-hydroxyatomoxetine, which is glucuronidated. 4-Hydroxyatomoxetine is equipotent to atomoxetine as an inhibitor of the norepinephrine transporter but circulates in plasma at much lower concentrations (1% of atomoxetine concentration in extensive metabolizers (EMs) and 0.1% of atomoxetine concentration in PMs). 4-Hydroxyatomoxetine is primarily formed by CYP2D6, but in PMs, 4-hydroxyatomoxetine is formed at a slower rate by several other cytochrome P450 enzymes. N-Desmethylatomoxetine is formed by CYP2C19 and other cytochrome P450 enzymes, but has substantially less pharmacological activity compared with atomoxetine and circulates in plasma at lower concentrations (5% of atomoxetine concentration in EMs and 45% of atomoxetine concentration in poor metabolizers (PMs)). The role of the polymorphic cytochrome p450 2D6 (CYP2D6) in the pharmacokinetics of atomoxetine hydrochloride [(-)-N-methyl-gamma-(2-methylphenoxy)benzenepropanamine hydrochloride; LY139603] has been documented following both single and multiple doses of the drug. In this study, the influence of the CYP2D6 polymorphism on the overall disposition and metabolism of a 20-mg dose of (14)C-atomoxetine was evaluated in CYP2D6 extensive metabolizer (EM; n = 4) and poor metabolizer (PM; n = 3) subjects under steady-state conditions. Atomoxetine was well absorbed from the gastrointestinal tract and cleared primarily by metabolism with the preponderance of radioactivity being excreted into the urine. In EM subjects, the majority of the radioactive dose was excreted within 24 hr, whereas in PM subjects the majority of the dose was excreted by 72 hr. The biotransformation of atomoxetine was similar in all subjects undergoing aromatic ring hydroxylation, benzylic oxidation, and N-demethylation with no CYP2D6 phenotype-specific metabolites. The primary oxidative metabolite of atomoxetine was 4-hydroxyatomoxetine, which was subsequently conjugated forming 4-hydroxyatomoxetine-O-glucuronide. Due to the absence of CYP2D6 activity, the systemic exposure to radioactivity was prolonged in PM subjects (t(1/2) = 62 hr) compared with EM subjects (t(1/2) = 18 hr). In EM subjects, atomoxetine (t(1/2) = 5 hr) and 4-hydroxyatomoxetine-O-glucuronide (t(1/2) = 7 hr) were the principal circulating species, whereas atomoxetine (t(1/2) = 20 hr) and N-desmethylatomoxetine (t(1/2) = 33 hr) were the principal circulating species in PM subjects. Although differences were observed in the excretion and relative amounts of metabolites formed, the primary difference observed between EM and PM subjects was the rate at which atomoxetine was biotransformed to 4-hydroxyatomoxetine. PMID:12485958 Atomoxetine is excreted primarily as 4-hydroxyatomoxetine-O-glucuronide, mainly in the urine (greater than 80% of the dose) and to a lesser extent in the feces (less than 17% of the dose). Only a small fraction of the Strattera dose is excreted as unchanged atomoxetine (less than 3% of the dose), indicating extensive biotransformation. Atomoxetine is primarily metabolized by the CYP2D6 pathway to 4-hydroxyatomoxetine. 4-Hydroxyatomoxetine is equipotent to atomoxetine as an inhibitor of the norepinephrine transporter but circulates in plasma at much lower concentrations (1% of atomoxetine concentration in EMs and 0.1% of atomoxetine concentration in PMs). Half Life: 5 hours The plasma elimination half life in normal (extensive) metabolizers is about 5 hours. In person who are poor metabolizers (7% of whites and 2% of blacks), the drug plasma levels are much higher and the plasma elimination half life is 24 hours. Mean apparent plasma clearance of atomoxetine after oral administration in adult extensive metabolizers (EMs) is 0.35 L/hr/kg and the mean half-life is 5.2 hours. Following oral administration of atomoxetine to poor metabolizers (PMs), mean apparent plasma clearance is 0.03 L/hr/kg and mean half-life is 21.6 hours. For PMs, AUC of atomoxetine is approximately 10-fold and Css, max is about 5-fold greater than EMs. The elimination half-life of 4-hydroxyatomoxetine is similar to that of N-desmethylatomoxetine (6 to 8 hours) in EM subjects, while the half-life of N-desmethylatomoxetine is much longer in PM subjects (34 to 40 hours). ... In extensive metabolizers, atomoxetine has a plasma half-life of 5.2 hours, while in poor metabolizers, atomoxetine has a plasma half-life of 21.6 hours. ... PMID:15910008 ... Twenty-one cytochrome P450 2D6 extensive metabolizer patients participated in these single-dose and steady-state pharmacokinetic evaluations. Atomoxetine was rapidly absorbed, with peak plasma concentrations occurring 1 to 2 hours after dosing. Half-life averaged 3.12 and 3.28 hours after a single dose and at steady state, respectively. ... Rat pharmacokinetics [4]: - Oral administration (10 mg/kg): Cmax = 85 ng/mL, Tmax = 1.5 hours, t1/2 = 3.2 hours, oral bioavailability (F) = 75%, CL = 15 mL/min/kg, Vd = 4.8 L/kg [4] - Intravenous administration (2 mg/kg): Cmax = 62 ng/mL, t1/2 = 2.8 hours, CL = 16 mL/min/kg [4] - Dog pharmacokinetics [4]: - Oral administration (10 mg/kg): Cmax = 68 ng/mL, Tmax = 2 hours, t1/2 = 4.5 hours, F = 68% [4] - Human pharmacokinetics [4]: - Healthy volunteers (n=12) oral administration (40 mg): Cmax = 80 ng/mL, Tmax = 1.8 hours, t1/2 = 5.2 hours, F = 60% (extensive metabolizers, CYP2D6 normal); t1/2 = 21 hours in poor CYP2D6 metabolizers [4] - Metabolism: Atomoxetine HCl is primarily metabolized by CYP2D6 in humans (accounts for ~70% of metabolism); major metabolite is 4-hydroxyatomoxetine (inactive). In CYP2D6 poor metabolizers, metabolism shifts to CYP1A2 (~40%) and CYP2C19 (~30%) [4] - Excretion: ~80% of dose excreted in urine (10% as unchanged drug, 70% as metabolites) over 48 hours; ~15% excreted in feces [4] |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Atomoxetine, as Strattera, is indicated for the treatment of Attention-Deficit/Hyperactivity Disorder (ADHD). HUMAN EXPOSURE AND TOXICITY: Atomoxetine increased the risk of suicidal ideation in short-term studies in children or adolescents with ADHD. Symptoms accompanying acute and chronic overdoses of atomoxetine include gastrointestinal symptoms, somnolence, dizziness, tremor, abnormal behavior, hyperactivity, agitation, and signs and symptoms consistent with mild to moderate sympathetic nervous system activation (e.g., tachycardia, blood pressure increased, mydriasis, dry mouth). Less commonly, there have been reports of QT prolongation and mental changes, including disorientation and hallucinations. Atomoxetine may cause clinically significant hepatotoxicity either by metabolic idiosyncrasy or by inducing autoimmune hepatitis. There have been fatalities reported involving a mixed ingestion overdose of Strattera and at least one other drug. Sudden deaths, stroke, and myocardial infarction have been reported in both children and adults with structural cardiac abnormalities or other serious heart problems. ANIMAL STUDIES: The median lethal oral dose of atomoxetine hydrochloride in animals was estimated to be 25 mg/kg for cats, >37.5 mg/kg for dogs, and 0.190 mg/kg in rats and mice. Premonitory signs of toxicity following single oral doses of atomoxetine in animals included mydriasis and reduced pupillary light reflex, mucoid stools, salivation, vomiting, ataxia, tremors, myoclonic jerking, and convulsions. Chronic toxicity studies of up to 1 year were conducted in adult rats and dogs. There was no major target organ toxicity observed in dogs given oral doses up to 16 mg/kg/day or in rats given atomoxetine in the diet at time-weighted average doses up to 47 mg/kg/day. These doses are 4-5 times the maximum recommended daily oral dose in adults. Mild hepatic effects, characterized by mottling and pallor of the liver, increased relative liver weights, hepatocellular vacuolation, and slightly increased serum ALT values, occurred in male rats given time weighted average doses >/= 14 mg/kg/day. No hepatic effects were observed in dogs. Clinical signs of mydriasis, reduced pupillary light reflex, emesis, and tremors were observed in dogs, and these effects were minimal in adult dogs given >/= 8 mg/kg/day. No evidence of drug-associated teratogenicity or retarded fetal development was produced in rabbits or rats administered atomoxetine hydrochloride throughout organogenesis at oral doses up to 100 mg/kg/day and 150 mg/kg/day (13 times the maximum recommended daily oral dose in adults). In a rat fertility study, decreased pup weight and survival was observed, predominantly during the first week postpartum following maternal dietary atomoxetine timeweighted average doses of 23 mg/kg/day or higher. Atomoxetine hydrochloride was negative in a battery of genotoxicity studies that included a reverse point mutation assay (Ames Test), an in vitro mouse lymphoma assay, a chromosomal aberration test in Chinese hamster ovary cells, an unscheduled DNA synthesis test in rat hepatocytes, and an in vivo micronucleus test in mice. However, there was a slight increase in the percentage of Chinese hamster ovary cells with diplochromosomes, suggesting endoreduplication (numerical aberration). Atomoxetine hydrochloride was not carcinogenic in rats and mice when given in the diet for 2 years at time-weighted average doses up to 47 and 458 mg/kg/day, respectively. The precise mechanism by which atomoxetine produces its therapeutic effects in Attention-Deficit/Hyperactivity Disorder (ADHD) is unknown, but is thought to be related to selective inhibition of the pre-synaptic norepinephrine transporter, as determined through in-vitro studies. Atomoxetine appears to have minimal affinity for other noradrenergic receptors or for other neurotransmitter transporters or receptors. Hepatotoxicity Atomoxetine has been linked to serum aminotransferase elevations in a small proportion of patients (~0.5%). More importantly, there have been several reports of clinically apparent acute liver injury due to atomoxetine. The onset of injury was within 3 to 12 weeks of starting the medication. The typical pattern of serum enzyme elevations was hepatocellular with marked increases in serum aminotransferase levels (often >20 times upper limit of normal) and clinical features that resembled acute viral hepatitis. Most cases have been self-limited, but instances of acute liver failure sometimes requiring emergency liver transplantation have been reported. Immunoallergic features were not found, but several patients with acute injury had antinuclear antibody and at least one patient had other features that resembled autoimmune hepatitis (with typical liver histology and high levels of immunoglobulins in serum). Likelihood score: C (probable cause of clinically apparent liver injury). View MoreEffects During Pregnancy and Lactation◉ Summary of Use during Lactation There is no published experience with atomoxetine during breastfeeding, although reports from the manufacturer found no serious adverse effects in two breastfed infants. If the mother of an older infant requires atomoxetine, it is not a reason to discontinue breastfeeding, but until more data become available, an alternate drug may be preferred, especially while nursing a newborn or preterm infant. Monitor breastfed infants for excess sedation. ◉ Effects in Breastfed Infants The author of a review article reported that the manufacturer of atomoxetine (Eli Lilly and Co.) had reports of 2 infants who slept longer than usual after being breastfed by mothers who were taking atomoxetine. Neither of the infants experienced any serious adverse events. Dosages, duration of maternal therapy, infant age and extent of breastfeeding were not provided. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. ◈ What is atomoxetine? Atomoxetine is a mediation that has been approved to treat attention deficit hyperactivity disorder (ADHD). It belongs to a class of medications known as norepinephrine reuptake inhibitors. A brand name for atomoxetine is Strattera®.Sometimes when people find out they are pregnant, they think about changing how they take their medication, or stopping their medication altogether. However, it is important to talk with your healthcare providers before making any changes to how you take this medication. Your healthcare providers can talk with you about the benefits of treating your condition and the risks of untreated illness during pregnancy. ◈ I take atomoxetine, and I was told that I am a poor/slow metabolizer. What does that mean for my pregnancy? Some people metabolize atomoxetine slower than others. People who are slow metabolizers might have higher levels of the medication in their blood. It is not known if this could affect a pregnancy differently than people who metabolize the medication more quickly. ◈ I take atomoxetine. Can it make it harder for me to get pregnant? Studies have not been done in humans to see if atomoxetine could make it harder to get pregnant. Animal studies did not show a change in fertility. ◈ Does taking atomoxetine increase the chance for miscarriage? Miscarriage is common and can occur in any pregnancy for many different reasons. Studies have not been done to see if atomoxetine could increase the chance of miscarriage. ◈ Does taking atomoxetine increase the chance of birth defects? Every pregnancy starts out with a 3-5% chance of having a birth defect. This is called the background risk. Atomoxetine has not been well studied for use during pregnancy. Four human studies have not suggested a greater chance for birth defects. Most of these studies used a prescription database to see who had a prescription for atomoxetine during their pregnancy. This cannot tell us if that person took atomoxetine during their pregnancy. When looking at doses typically used by humans, animal studies did not suggest an increased chance for birth defects. With levels higher than those used with human treatment, there is some question of a higher chance for birth defects. It is not known if this information would apply to people who are considered poor metabolizers. ◈ Does taking atomoxetine in pregnancy increase the chance of other pregnancy-related problems? It is not known if atomoxetine can cause other pregnancy-related problems. One study of 453 people who filled a prescription for atomoxetine during the first 20 weeks of pregnancy showed no increased chance for placental abruption (when the placenta pulls away from the wall of the uterus before labor starts), smallness for gestational age, preterm birth (birth before 37 weeks of pregnancy), or preeclampsia (dangerously high blood pressure). ◈ Does taking atomoxetine in pregnancy cause long-term problems in behavior or learning for the childy? Studies have not been done to see if atomoxetine can cause behavior or learning issues for the child. ◈ Breastfeeding while taking atomoxetine: There are no studies on the use of atomoxetine while breastfeeding. If breastfeeding and taking the medication, and you suspect the baby has any symptoms such as excess sedation, contact the child’s healthcare provider. Be sure to talk to your healthcare providers about all your breastfeeding questions. ◈ If a male takes atomoxetine, could it affect fertility (ability to get partner pregnant) or increase the chance of birth defects? Studies have not been done to see if atomoxetine could affect male fertility or increase the chance of birth defects. In general, exposures that fathers or sperm donors have are unlikely to increase the risks to a pregnancy. For more information, please see the MotherToBaby fact sheet Paternal Exposures at https://mothertobaby.org/fact-sheets/paternal-exposures-pregnancy/. Exposure Routes Atomoxetine is rapidly absorbed after oral administration, with absolute bioavailability of about 63% in EMs and 94% in PMs. Drugs that elevate gastric pH (magnesium hydroxide/aluminum hydroxide, omeprazole) have no effect on atomoxetine bioavailability. Absorption is minimally affected by food. Symptoms The most commonly reported symptoms accompanying acute and chronic overdoses are somnolence, agitation, hyperactivity, abnormal behavior, and gastrointestinal symptoms. Treatment An airway should be established. Monitoring of cardiac and vital signs is recommended, along with appropriate symptomatic and supportive measures. Gastric lavage may be indicated if performed soon after ingestion. Activated charcoal may be useful in limiting absorption. Because atomoxetine is highly protein-bound, dialysis is not likely to be useful in the treatment of overdose. (L1712) L1712: RxList: The Internet Drug Index (2009). http://www.rxlist.com/ Interactions Strattera should be administered with caution to patients being treated with systemically-administered (oral or intravenous) albuterol (or other beta2 agonists) because the action of albuterol on the cardiovascular system can be potentiated resulting in increases in heart rate and blood pressure. Albuterol (600 mcg iv over 2 hours) induced increases in heart rate and blood pressure. These effects were potentiated by atomoxetine (60 mg BID for 5 days) and were most marked after the initial coadministration of albuterol and atomoxetine. However, these effects on heart rate and blood pressure were not seen in another study after the coadministration with inhaled dose of albuterol (200-800 ug) and atomoxetine (80 mg QD for 5 days) in 21 healthy Asian subjects who were excluded for poor metabolizer status. The manufacturer states that atomoxetine is contraindicated in patients currently receiving or having recently received (i.e., within 2 weeks) monoamine oxidase (MAO) inhibitor therapy. In addition, at least 2 weeks should elapse after discontinuing atomoxetine before initiating MAO inhibitor therapy. Severe, potentially fatal, reactions (including hyperthermia, rigidity, myoclonus, autonomic instability with possible rapid fluctuations of vital signs, and mental status changes that include extreme agitation progressing to delirium and coma) have been reported in patients receiving other drugs that affect brain monoamine concentrations concomitantly with MAO inhibitor therapy. Protein Binding At therapeutic concentrations, 98.7% of plasma atomoxetine is bound to protein, with 97.5% of that being bound to albumin, followed by alpha-1-acid glycoprotein and immunoglobulin G. Acute toxicity [4]: - Mouse oral LD50 = 350 mg/kg; rats oral LD50 = 280 mg/kg. Signs of acute toxicity included sedation, ataxia, and decreased locomotion, with full recovery within 24 hours at sub-LD50 doses [4] - Rat subacute toxicity (28 days, oral 10 mg/kg, 30 mg/kg): No significant changes in body weight, food intake, or hematological parameters (WBC, RBC, platelets). Serum ALT/AST levels were slightly elevated (~15%) at 30 mg/kg but remained within normal range; no histopathological lesions in liver/kidney [4] - Plasma protein binding: 98% in human plasma (equilibrium dialysis), 97% in rat plasma, 96% in dog plasma; binding not affected by pH (6.5–8.0) or plasma concentration (10–1000 ng/mL) [4] - Drug-drug interactions [4]: - Co-administration with CYP2D6 inhibitors (e.g., paroxetine) increases Atomoxetine HCl plasma concentrations by ~3-fold in extensive metabolizers [4] |
| 参考文献 |
|
| 其他信息 |
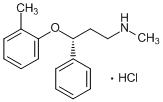
Atomoxetine hydrochloride is the hydrochloride salt of atomoxetine. It has a role as an antidepressant and an adrenergic uptake inhibitor. It contains an atomoxetine.
Atomoxetine Hydrochloride is the hydrochloride salt of atomoxetine, a phenoxy-3-propylamine derivative and selective non-stimulant, norepinephrine reuptake inhibitor with cognitive-enhancing activity. Although its precise mechanism of action is unknown, atomoxetine appears to selectively inhibit the pre-synaptic norepinephrine transporter, resulting in inhibition of the presynaptic reabsorption of norepinephrine and prolongation of norepinephrine activity in the synaptic cleft. The effect on cognitive brain function may result in improved attention and decreased impulsivity and activity levels. A propylamine derivative and selective ADRENERGIC UPTAKE INHIBITOR that is used in the treatment of ATTENTION DEFICIT HYPERACTIVITY DISORDER. See also: Atomoxetine (has active moiety). Drug Indication Treatment of Attention Deficit Hyperactivity Disorder (ADHD) Atomoxetine HCl (formerly tomoxetine) is a selective norepinephrine reuptake inhibitor (SNRI) initially developed as an antidepressant but later approved for the treatment of attention deficit/hyperactivity disorder (ADHD) in children, adolescents, and adults [1][3] - Mechanism of action in ADHD: By inhibiting NET, Atomoxetine HCl increases extracellular NE levels in the prefrontal cortex (PFC), enhancing PFC-mediated executive functions (attention, impulse control, working memory). It also modestly increases PFC DA levels (via NE-induced DAT inhibition), which contributes to ADHD efficacy [3] - Clinical dosage: Recommended oral dose for ADHD is 0.5–1.4 mg/kg/day (once daily or divided into two doses); dose adjustments are required for CYP2D6 poor metabolizers (maximum 40 mg/day) [4] - Literature [2] identifies a novel off-target effect: state/use-dependent VGSC inhibition, which may contribute to its mild side effects (e.g., dizziness, palpitations) but is not directly related to ADHD efficacy [2] - Unlike stimulant ADHD medications (e.g., methylphenidate), Atomoxetine HCl has no abuse potential (due to lack of DAT inhibition) and is classified as a non-controlled substance [3][4] |
| 分子式 |
C17H22CLNO
|
|
|---|---|---|
| 分子量 |
291.82
|
|
| 精确质量 |
291.138
|
|
| 元素分析 |
C, 69.97; H, 7.60; Cl, 12.15; N, 4.80; O, 5.48
|
|
| CAS号 |
82248-59-7
|
|
| 相关CAS号 |
Atomoxetine; 83015-26-3; Atomoxetine-d5 hydrochloride
|
|
| PubChem CID |
54840
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 沸点 |
389ºC at 760 mmHg
|
|
| 熔点 |
167-169ºC
|
|
| 闪点 |
164.1ºC
|
|
| LogP |
4.917
|
|
| tPSA |
21.26
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
2
|
|
| 可旋转键数目(RBC) |
6
|
|
| 重原子数目 |
20
|
|
| 分子复杂度/Complexity |
237
|
|
| 定义原子立体中心数目 |
1
|
|
| SMILES |
[C@H](C1C=CC=CC=1)(CCNC)OC1C=CC=CC=1C.Cl
|
|
| InChi Key |
LUCXVPAZUDVVBT-UNTBIKODSA-N
|
|
| InChi Code |
InChI=1S/C17H21NO.ClH/c1-14-8-6-7-11-16(14)19-17(12-13-18-2)15-9-4-3-5-10-15;/h3-11,17-18H,12-13H2,1-2H3;1H/t17-;/m1./s1
|
|
| 化学名 |
(3R)-N-methyl-3-(2-methylphenoxy)-3-phenylpropan-1-amine;hydrochloride
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (8.57 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (8.57 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (8.57 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 8.33 mg/mL (28.54 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶 (<60°C). 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.4268 mL | 17.1338 mL | 34.2677 mL | |
| 5 mM | 0.6854 mL | 3.4268 mL | 6.8535 mL | |
| 10 mM | 0.3427 mL | 1.7134 mL | 3.4268 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Comparison of Lisdexamfetamine Dimesylate With Atomoxetine HCl in Attention-Deficit/Hyperactivity Disorder (ADHD) Subjects With an Inadequate Response to Methylphenidate
CTID: NCT01106430
Phase: Phase 3 Status: Completed
Date: 2021-06-11
 |
|---|
  |
 |