| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| Other Sizes |
| 靶点 |
Transporters fpr norepinephrine (NE), serotonin (5-HT) and dopamine (DA); SNRI/selective noradrenaline reuptake inhibitor
|
|---|---|
| 体外研究 (In Vitro) |
人心脏钠通道 (hNav1.5) 与托莫西汀(1-100 µM;0.5-20 秒;tsA201 细胞)以状态和剂量依赖性方式相互作用 [2]。
托莫西汀是一种苯氧基-3-丙胺衍生物,也是一种具有认知增强活性的选择性非兴奋剂去甲肾上腺素再摄取抑制剂。尽管其确切的作用机制尚不清楚,但托莫西汀似乎选择性地抑制了突触前去甲肾上腺素转运蛋白,从而抑制了去甲肾上腺素的突触前重吸收,延长了突触间隙中的去甲肾上腺素活性。对认知脑功能的影响可能会导致注意力提高,冲动和活动水平降低。 |
| 体内研究 (In Vivo) |
Tomoxetine(0.3-3 mg/kg;腹腔注射;0-4 小时;雄性 Sprague-Dawley 大鼠)可将细胞外去甲肾上腺素和多巴胺增加 3 倍,并增加 Fos 的细胞内表达[1]。托莫西汀(0.1-5 mg/kg;腹腔注射和口服;持续14天;自发性高血压大鼠)可以改善大鼠ADHD相关行为[3]。
托莫西汀(ATX)是一种常用的非兴奋剂治疗注意缺陷多动障碍(ADHD)的药物。它主要作用是增加去甲肾上腺素水平;然而,在较高剂量下,它可以增加多巴胺水平。迄今为止,还没有研究口服ATX对最常用的ADHD模型——自发性高血压大鼠(SHR)的影响。本研究旨在描述去甲肾上腺素选择性(0.15 mg/kg)和非选择性(0.3 mg/kg)剂量对SHR行为测量的影响。首先,我们研究了急性和慢性ATX对运动活动的影响,包括对苯丙胺的致敏和交叉致敏。其次,我们使用T迷宫延迟折扣范式测量了药物对冲动性的影响。我们发现ATX对运动活动没有影响,也没有证据表明有致敏或交叉致敏。此外,T迷宫性能没有差异,表明这些剂量对冲动性没有影响。行为敏感性的缺失支持了之前关于所服用剂量相对于精神兴奋剂具有更高安全性的说法。对冲动性也没有影响;然而,我们认为这被SHR特有的压力所混淆。讨论了对未来研究的影响、SHR的行为评估及其作为ADHD模型的使用[1]。 |
| 酶活实验 |
托莫西汀是一种神经活性药物,已被批准用于治疗注意力缺陷/多动障碍(ADHD)。它主要被称为去甲肾上腺素转运蛋白的高亲和力阻断剂,因此其应用导致不同脑区相应神经递质水平的增加。然而,用于获得临床效果的浓度远高于阻断转运系统所需的浓度。因此,可能会出现脱靶效应。通过这种方式,我们之前已经确定托莫西汀是NMDA受体的阻断剂。由于许多精神药物会导致心源性猝死,我们现在测试了托莫西汀在临床相关浓度下是否也与心肌类型的电压门控钠通道相互作用的假设。通过膜片钳技术在人胚胎肾细胞中异质表达的人心肌钠通道(hNav1.5)上进行电生理实验。托莫西汀以状态和使用依赖的方式抑制钠通道。托莫西汀对hNav1.5的静息状态只有微弱的亲和力(Kr:∼120µM)。托莫西汀的疗效随着膜去极化而显著增加,表明失活状态是一个重要靶点。这种药物的一个标志是其缓慢的相互作用。通过使用不同的实验设置,我们得出结论,这种相互作用发生在缓慢失活状态,也发生在快速失活状态的缓慢动力学中。半最大有效浓度(2-3µM)完全在治疗患者血浆中的浓度范围内。托莫西汀也与开放通道相互作用。然而,这种相互作用的速度不足以加速快速失活的时间常数。然而,当使用失活缺陷的hNav1.5_I408W_L409C_A410W突变体时,我们发现持续的晚期电流在约3µM托莫西汀时被阻断了一半。相互作用很可能是通过局部麻醉剂结合位点发生的。托莫西汀抑制钠通道的浓度与治疗ADHD的浓度相似。由于其缓慢的相互作用和抑制晚期电流,它可能具有抗心律失常特性[2]。
|
| 动物实验 |
Animal/Disease Models: Male SD (SD (Sprague-Dawley)) rat [1]
Doses: 0.3, 1 and 3 mg/kg Route of Administration: intraperitoneal (ip) injection; 4 hrs (hrs (hours)) Experimental Results: The number of cells expressing Fos-like immunoreactivity increased 3.7-fold in the PFC, extracellular Norepinephrine and dopamine increase 3-fold. Animal/Disease Models: Spontaneously hypertensive rat (SHR) [3] Doses: 0.1, 0.3, 1.25 and 5.0 mg/kg Route of Administration: intraperitoneal (ip) injection and oral administration; continued for 14 days Experimental Results: No effect on measurements of locomotor activity. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The pharmacokinetic profile of atomoxetine is highly dependent on cytochrome P450 2D6 genetic polymorphisms of the individual. A large fraction of the population (up to 10% of Caucasians and 2% of people of African descent and 1% of Asians) are poor metabolizers (PMs) of CYP2D6 metabolized drugs. These individuals have reduced activity in this pathway resulting in 10-fold higher AUCs, 5-fold higher peak plasma concentrations, and slower elimination (plasma half-life of 21.6 hours) of atomoxetine compared with people with normal CYP2D6 activity. Atomoxetine is rapidly absorbed after oral administration, with absolute bioavailability of about 63% in extensive metabolizers (EMs) and 94% in poor metabolizers (PMs). Mean maximal plasma concentrations (Cmax) are reached approximately 1 to 2 hours after dosing with a maximal concentration of 350 ng/ml with an AUC of 2 mcg.h/ml. Atomoxetine is excreted primarily as 4-hydroxyatomoxetine-O-glucuronide, mainly in the urine (greater than 80% of the dose) and to a lesser extent in the feces (less than 17% of the dose). Only a small fraction (less than 3%) of the atomoxetine dose is excreted as unchanged atomoxetine, indicating extensive biotransformation. The reported volume of distribution of oral atomoxetine was 1.6-2.6 L/kg. The steady-state volume of distribution of intravenous atomoxetine was approximately 0.85 L/kg. The clearance rate of atomoxetine depends the CYP2D6 genetic polymorphisms of the individual and can range of 0.27-0.67 L.h/kg. Steady-state volume of distribution (intravenous administration): 8.5 L/kg. Atomexetine distributes primarily into total body water; volume of distribution is similar across patient weight range after normalizing for body weight. Atomoxetine is rapidly absorbed after oral administration, with absolute bioavailability of about 63% in extensive metabolizers and 94% in poor metabolizers. Maximal plasma concentrations (Cmax) are reached approximately 1 to 2 hours after dosing. /MILK/ Atomoxetine and/or its metabolites are distributed into milk in rats; it is not known whether the drug is distributed into milk in humans. ... Atomoxetine has high aqueous solubility and biological membrane permeability that facilitates its rapid and complete absorption after oral administration. Absolute oral bioavailability ranges from 63 to 94%, which is governed by the extent of its first-pass metabolism. Three oxidative metabolic pathways are involved in the systemic clearance of atomoxetine: aromatic ring-hydroxylation, benzylic hydroxylation and N-demethylation. Aromatic ring-hydroxylation results in the formation of the primary oxidative metabolite of atomoxetine, 4-hydroxyatomoxetine, which is subsequently glucuronidated and excreted in urine. The formation of 4-hydroxyatomoxetine is primarily mediated by the polymorphically expressed enzyme cytochrome P450 (CYP) 2D6. This results in two distinct populations of individuals: those exhibiting active metabolic capabilities (CYP2D6 extensive metabolizers) and those exhibiting poor metabolic capabilities (CYP2D6 poor metabolizers) for atomoxetine. The oral bioavailability and clearance of atomoxetine are influenced by the activity of CYP2D6; nonetheless, plasma pharmacokinetic parameters are predictable in extensive and poor metabolizer patients. After single oral dose, atomoxetine reaches maximum plasma concentration within about 1-2 hours of administration. In extensive metabolizers, atomoxetine has a plasma half-life of 5.2 hours, while in poor metabolizers, atomoxetine has a plasma half-life of 21.6 hours. The systemic plasma clearance of atomoxetine is 0.35 and 0.03 L/h/kg in extensive and poor metabolizers, respectively. Correspondingly, the average steady-state plasma concentrations are approximately 10-fold higher in poor metabolizers compared with extensive metabolizers. Upon multiple dosing there is plasma accumulation of atomoxetine in poor metabolizers, but very little accumulation in extensive metabolizers. The volume of distribution is 0.85 L/kg, indicating that atomoxetine is distributed in total body water in both extensive and poor metabolizers. Atomoxetine is highly bound to plasma albumin (approximately 99% bound in plasma). Although steady-state concentrations of atomoxetine in poor metabolizers are higher than those in extensive metabolizers following administration of the same mg/kg/day dosage, the frequency and severity of adverse events are similar regardless of CYP2D6 phenotype.Atomoxetine administration does not inhibit or induce the clearance of other drugs metabolized by CYP enzymes. In extensive metabolizers, potent and selective CYP2D6 inhibitors reduce atomoxetine clearance; however, administration of CYP inhibitors to poor metabolizers has no effect on the steady-state plasma concentrations of atomoxetine. For more Absorption, Distribution and Excretion (Complete) data for ATOMOXETINE (8 total), please visit the HSDB record page. Metabolism / Metabolites Atomoxetine undergoes biotransformation primarily through the cytochrome P450 2D6 (CYP2D6) enzymatic pathway. People with reduced activity in the CYP2D6 pathway (also known as poor metabolizers or PMs) have higher plasma concentrations of atomoxetine compared with people with normal activity (also known as extensive metabolizers, or EMs). For PMs, the AUC of atomoxetine at steady-state is approximately 10-fold higher and Cmax is about 5-fold greater than for EMs. The major oxidative metabolite formed regardless of CYP2D6 status is 4-hydroxy-atomoxetine, which is rapidly glucuronidated. 4-Hydroxyatomoxetine is equipotent to atomoxetine as an inhibitor of the norepinephrine transporter, but circulates in plasma at much lower concentrations (1% of atomoxetine concentration in EMs and 0.1% of atomoxetine concentration in PMs). In individuals that lack CYP2D6 activity, 4-hydroxyatomoxetine is still the primary metabolite, but is formed by several other cytochrome P450 enzymes and at a slower rate. Another minor metabolite, N-Desmethyl-atomoxetine is formed by CYP2C19 and other cytochrome P450 enzymes, but has much less pharmacological activity than atomoxetine and lower plasma concentrations (5% of atomoxetine concentration in EMs and 45% of atomoxetine concentration in PMs). Atomoxetine is metabolized primarily through the CYP2D6 enzymatic pathway. People with reduced activity in this pathway (PMs) have higher plasma concentrations of atomoxetine compared with people with normal activity (EMs). For PMs, AUC of atomoxetine is approximately 10-fold and Css, max is about 5-fold greater than EMs. Laboratory tests are available to identify CYP2D6 PMs. The major oxidative metabolite formed, regardless of CYP2D6 status, is 4-hydroxyatomoxetine, which is glucuronidated. 4-Hydroxyatomoxetine is equipotent to atomoxetine as an inhibitor of the norepinephrine transporter but circulates in plasma at much lower concentrations (1% of atomoxetine concentration in extensive metabolizers (EMs) and 0.1% of atomoxetine concentration in PMs). 4-Hydroxyatomoxetine is primarily formed by CYP2D6, but in PMs, 4-hydroxyatomoxetine is formed at a slower rate by several other cytochrome P450 enzymes. N-Desmethylatomoxetine is formed by CYP2C19 and other cytochrome P450 enzymes, but has substantially less pharmacological activity compared with atomoxetine and circulates in plasma at lower concentrations (5% of atomoxetine concentration in EMs and 45% of atomoxetine concentration in poor metabolizers (PMs)). The role of the polymorphic cytochrome p450 2D6 (CYP2D6) in the pharmacokinetics of atomoxetine hydrochloride [(-)-N-methyl-gamma-(2-methylphenoxy)benzenepropanamine hydrochloride; LY139603] has been documented following both single and multiple doses of the drug. In this study, the influence of the CYP2D6 polymorphism on the overall disposition and metabolism of a 20-mg dose of (14)C-atomoxetine was evaluated in CYP2D6 extensive metabolizer (EM; n = 4) and poor metabolizer (PM; n = 3) subjects under steady-state conditions. Atomoxetine was well absorbed from the gastrointestinal tract and cleared primarily by metabolism with the preponderance of radioactivity being excreted into the urine. In EM subjects, the majority of the radioactive dose was excreted within 24 hr, whereas in PM subjects the majority of the dose was excreted by 72 hr. The biotransformation of atomoxetine was similar in all subjects undergoing aromatic ring hydroxylation, benzylic oxidation, and N-demethylation with no CYP2D6 phenotype-specific metabolites. The primary oxidative metabolite of atomoxetine was 4-hydroxyatomoxetine, which was subsequently conjugated forming 4-hydroxyatomoxetine-O-glucuronide. Due to the absence of CYP2D6 activity, the systemic exposure to radioactivity was prolonged in PM subjects (t(1/2) = 62 hr) compared with EM subjects (t(1/2) = 18 hr). In EM subjects, atomoxetine (t(1/2) = 5 hr) and 4-hydroxyatomoxetine-O-glucuronide (t(1/2) = 7 hr) were the principal circulating species, whereas atomoxetine (t(1/2) = 20 hr) and N-desmethylatomoxetine (t(1/2) = 33 hr) were the principal circulating species in PM subjects. Although differences were observed in the excretion and relative amounts of metabolites formed, the primary difference observed between EM and PM subjects was the rate at which atomoxetine was biotransformed to 4-hydroxyatomoxetine. Atomoxetine is excreted primarily as 4-hydroxyatomoxetine-O-glucuronide, mainly in the urine (greater than 80% of the dose) and to a lesser extent in the feces (less than 17% of the dose). Only a small fraction of the Strattera dose is excreted as unchanged atomoxetine (less than 3% of the dose), indicating extensive biotransformation. For more Metabolism/Metabolites (Complete) data for ATOMOXETINE (8 total), please visit the HSDB record page. Atomoxetine is primarily metabolized by the CYP2D6 pathway to 4-hydroxyatomoxetine. 4-Hydroxyatomoxetine is equipotent to atomoxetine as an inhibitor of the norepinephrine transporter but circulates in plasma at much lower concentrations (1% of atomoxetine concentration in EMs and 0.1% of atomoxetine concentration in PMs). Half Life: 5 hours Biological Half-Life The reported half-life depends on the CYP2D6 genetic polymorphisms of the individual and can range from 3 to 5.6 hours. The plasma elimination half life in normal (extensive) metabolizers is about 5 hours. In person who are poor metabolizers (7% of whites and 2% of blacks), the drug plasma levels are much higher and the plasma elimination half life is 24 hours. Mean apparent plasma clearance of atomoxetine after oral administration in adult extensive metabolizers (EMs) is 0.35 L/hr/kg and the mean half-life is 5.2 hours. Following oral administration of atomoxetine to poor metabolizers (PMs), mean apparent plasma clearance is 0.03 L/hr/kg and mean half-life is 21.6 hours. For PMs, AUC of atomoxetine is approximately 10-fold and Css, max is about 5-fold greater than EMs. The elimination half-life of 4-hydroxyatomoxetine is similar to that of N-desmethylatomoxetine (6 to 8 hours) in EM subjects, while the half-life of N-desmethylatomoxetine is much longer in PM subjects (34 to 40 hours). ... In extensive metabolizers, atomoxetine has a plasma half-life of 5.2 hours, while in poor metabolizers, atomoxetine has a plasma half-life of 21.6 hours. ... ... Twenty-one cytochrome P450 2D6 extensive metabolizer patients participated in these single-dose and steady-state pharmacokinetic evaluations. Atomoxetine was rapidly absorbed, with peak plasma concentrations occurring 1 to 2 hours after dosing. Half-life averaged 3.12 and 3.28 hours after a single dose and at steady state, respectively. ... |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Atomoxetine, as Strattera, is indicated for the treatment of Attention-Deficit/Hyperactivity Disorder (ADHD). HUMAN EXPOSURE AND TOXICITY: Atomoxetine increased the risk of suicidal ideation in short-term studies in children or adolescents with ADHD. Symptoms accompanying acute and chronic overdoses of atomoxetine include gastrointestinal symptoms, somnolence, dizziness, tremor, abnormal behavior, hyperactivity, agitation, and signs and symptoms consistent with mild to moderate sympathetic nervous system activation (e.g., tachycardia, blood pressure increased, mydriasis, dry mouth). Less commonly, there have been reports of QT prolongation and mental changes, including disorientation and hallucinations. Atomoxetine may cause clinically significant hepatotoxicity either by metabolic idiosyncrasy or by inducing autoimmune hepatitis. There have been fatalities reported involving a mixed ingestion overdose of Strattera and at least one other drug. Sudden deaths, stroke, and myocardial infarction have been reported in both children and adults with structural cardiac abnormalities or other serious heart problems. ANIMAL STUDIES: The median lethal oral dose of atomoxetine hydrochloride in animals was estimated to be 25 mg/kg for cats, >37.5 mg/kg for dogs, and 0.190 mg/kg in rats and mice. Premonitory signs of toxicity following single oral doses of atomoxetine in animals included mydriasis and reduced pupillary light reflex, mucoid stools, salivation, vomiting, ataxia, tremors, myoclonic jerking, and convulsions. Chronic toxicity studies of up to 1 year were conducted in adult rats and dogs. There was no major target organ toxicity observed in dogs given oral doses up to 16 mg/kg/day or in rats given atomoxetine in the diet at time-weighted average doses up to 47 mg/kg/day. These doses are 4-5 times the maximum recommended daily oral dose in adults. Mild hepatic effects, characterized by mottling and pallor of the liver, increased relative liver weights, hepatocellular vacuolation, and slightly increased serum ALT values, occurred in male rats given time weighted average doses >/= 14 mg/kg/day. No hepatic effects were observed in dogs. Clinical signs of mydriasis, reduced pupillary light reflex, emesis, and tremors were observed in dogs, and these effects were minimal in adult dogs given >/= 8 mg/kg/day. No evidence of drug-associated teratogenicity or retarded fetal development was produced in rabbits or rats administered atomoxetine hydrochloride throughout organogenesis at oral doses up to 100 mg/kg/day and 150 mg/kg/day (13 times the maximum recommended daily oral dose in adults). In a rat fertility study, decreased pup weight and survival was observed, predominantly during the first week postpartum following maternal dietary atomoxetine timeweighted average doses of 23 mg/kg/day or higher. Atomoxetine hydrochloride was negative in a battery of genotoxicity studies that included a reverse point mutation assay (Ames Test), an in vitro mouse lymphoma assay, a chromosomal aberration test in Chinese hamster ovary cells, an unscheduled DNA synthesis test in rat hepatocytes, and an in vivo micronucleus test in mice. However, there was a slight increase in the percentage of Chinese hamster ovary cells with diplochromosomes, suggesting endoreduplication (numerical aberration). Atomoxetine hydrochloride was not carcinogenic in rats and mice when given in the diet for 2 years at time-weighted average doses up to 47 and 458 mg/kg/day, respectively. The precise mechanism by which atomoxetine produces its therapeutic effects in Attention-Deficit/Hyperactivity Disorder (ADHD) is unknown, but is thought to be related to selective inhibition of the pre-synaptic norepinephrine transporter, as determined through in-vitro studies. Atomoxetine appears to have minimal affinity for other noradrenergic receptors or for other neurotransmitter transporters or receptors. Interactions Strattera should be administered with caution to patients being treated with systemically-administered (oral or intravenous) albuterol (or other beta2 agonists) because the action of albuterol on the cardiovascular system can be potentiated resulting in increases in heart rate and blood pressure. Albuterol (600 mcg iv over 2 hours) induced increases in heart rate and blood pressure. These effects were potentiated by atomoxetine (60 mg BID for 5 days) and were most marked after the initial coadministration of albuterol and atomoxetine. However, these effects on heart rate and blood pressure were not seen in another study after the coadministration with inhaled dose of albuterol (200-800 ug) and atomoxetine (80 mg QD for 5 days) in 21 healthy Asian subjects who were excluded for poor metabolizer status. The manufacturer states that atomoxetine is contraindicated in patients currently receiving or having recently received (i.e., within 2 weeks) monoamine oxidase (MAO) inhibitor therapy. In addition, at least 2 weeks should elapse after discontinuing atomoxetine before initiating MAO inhibitor therapy. Severe, potentially fatal, reactions (including hyperthermia, rigidity, myoclonus, autonomic instability with possible rapid fluctuations of vital signs, and mental status changes that include extreme agitation progressing to delirium and coma) have been reported in patients receiving other drugs that affect brain monoamine concentrations concomitantly with MAO inhibitor therapy. Potential pharmacokinetic interaction (decreased metabolism of atomoxetine) when atomoxetine is used concomitantly with drugs that inhibit the activity of the cytochrome P-450 2D6 (CYP2D6) isoenzyme. Inhibitors of CYP2D6 may increase plasma concentrations of atomoxetine in patients with the extensive-metabolizer phenotype to such an extent that plasma concentrations of the drug are similar to those achieved in poor metabolizers. When atomoxetine is used concomitantly with potent CYP2D6 inhibitors (e.g., paroxetine, fluoxetine, quinidine), or in patients with poor-metabolizer phenotypes of the CYP2D6 isoenzyme, the manufacturer states that dosage adjustment of atomoxetine should be considered. However, in vitro studies suggest that concomitant use of atomoxetine with CYP2D6 inhibitors will not increase plasma concentrations of atomoxetine in patients with the poor-metabolizer phenotype. Potential pharmacologic interaction (increased hypertensive effects) with concomitant use of pressor agents (e.g., dopamine, dobutamine) and atomoxetine. Use with caution. For more Interactions (Complete) data for ATOMOXETINE (6 total), please visit the HSDB record page. |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Adrenergic Uptake Inhibitors /CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Atomoxetine is included in the database. Strattera is indicated for the treatment of Attention-Deficit/Hyperactivity Disorder (ADHD). /Included in US product labeling/ /EXPL THER/ /The purpose of this study was/ to observe the efficacy and safety of atomoxetine hydrochloride in children with narcolepsy. Totally 66 patients with narcolepsy who were conformed international classification of sleep disturbances (ICSD-2) diagnostic criteria treated with atomoxetine hydrochloride seen from November 2010 to December 2014 were enrolled into this study, 42 of them were male and 24 female, mean age of onset was 7.5 years (3.75-13.00 years), mean duration before diagnosis was 1.75 years (0.25-5.00 years). Complete blood count, liver and kidney function, multiple sleep latency test (MSLT), polysomnography (PGS), neuroimaging and electroencephalography (EEG) were performed for each patient. For some of the children HLA-DR2 gene and serum markers of infection were tested. The 66 cases were followed up from 2 to 49 months (average 18 months) to observe the clinical efficacy and adverse reactions. In 62 cases excessive daytime sleepiness was improved, in 11 cases (16.7%) it was controlled (16.7%), in 29 cases (43.9%) the treatment was obviously effective and in 22 (33.3%) it was effective; cataplexy occurred in 54 cases, in 18 (33.3%) it was controlled, in 19 (35.2%) the treatment was obviously effective and in 10 (18.5%) effective; night sleep disorders existed in 55 cases, in 47 cases it was improved, in 14 (25.5%) it was controlled, in 20 (36.4%) the treatment was obviously effective and in 13 (23.6%) effective; hypnagogic or hypnopompic hallucination was present in 13 cases, in only 4 these symptoms were controlled. Sleep paralysis existed in 4 cases, it was controlled in only 1 case. In 18 cases attention and learning efficiency improved.Anorexia occurred in 18 cases, mood disorder in 5 cases, depression in 2 cases, nocturia, muscle tremors, involuntary tongue movement each occurred in 1 case. P-R interval prolongation and atrial premature contraction were found in 1 case. Atomoxetine hydrochloride showed good effects in patients with narcolepsy on excessive daytime sleepiness, cataplexy and night sleep disorders, the effects on hallucinations and sleep paralysis were not significant. Adverse reactions were slight, anorexia and mood disorder were common. As a non-central nervous system stimulant, atomoxetine hydrochloride does not induce drug dependence and has no prescription limits; it has good tolerability, safety and effectiveness, it can be a good alternative in treatment of children with narcolepsy. For more Therapeutic Uses (Complete) data for ATOMOXETINE (6 total), please visit the HSDB record page. Drug Warnings /BOXED WARNING/ WARNING: SUICIDAL IDEATION IN CHILDREN AND ADOLESCENTS. Strattera (atomoxetine) increased the risk of suicidal ideation in short-term studies in children or adolescents with Attention-Deficit/Hyperactivity Disorder (ADHD). Anyone considering the use of Strattera in a child or adolescent must balance this risk with the clinical need. Co-morbidities occurring with ADHD may be associated with an increase in the risk of suicidal ideation and/or behavior. Patients who are started on therapy should be monitored closely for suicidality (suicidal thinking and behavior), clinical worsening, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Strattera is approved for ADHD in pediatric and adult patients. Strattera is not approved for major depressive disorder. Pooled analyses of short-term (6 to 18 weeks) placebo-controlled trials of Strattera in children and adolescents (a total of 12 trials involving over 2200 patients, including 11 trials in ADHD and 1 trial in enuresis) have revealed a greater risk of suicidal ideation early during treatment in those receiving Strattera compared to placebo. The average risk of suicidal ideation in patients receiving Strattera was 0.4% (5/1357 patients), compared to none in placebo-treated patients (851 patients). No suicides occurred in these trials. Postmarketing reports indicate that Strattera can cause severe liver injury. Although no evidence of liver injury was detected in clinical trials of about 6000 patients, there have been rare cases of clinically significant liver injury that were considered probably or possibly related to Strattera use in postmarketing experience. Rare cases of liver failure have also been reported, including a case that resulted in a liver transplant. Because of probable underreporting, it is impossible to provide an accurate estimate of the true incidence of these reactions. Reported cases of liver injury occurred within 120 days of initiation of atomoxetine in the majority of cases and some patients presented with markedly elevated liver enzymes [>20 X upper limit of normal (ULN)], and jaundice with significantly elevated bilirubin levels (>2 X ULN), followed by recovery upon atomoxetine discontinuation. In one patient, liver injury, manifested by elevated hepatic enzymes up to 40 X ULN and jaundice with bilirubin up to 12 X ULN, recurred upon rechallenge, and was followed by recovery upon drug discontinuation, providing evidence that Strattera likely caused the liver injury. Such reactions may occur several months after therapy is started, but laboratory abnormalities may continue to worsen for several weeks after drug is stopped. The patient described above recovered from his liver injury, and did not require a liver transplant. Strattera should be discontinued in patients with jaundice or laboratory evidence of liver injury, and should not be restarted. Laboratory testing to determine liver enzyme levels should be done upon the first symptom or sign of liver dysfunction (e.g., pruritus, dark urine, jaundice, right upper quadrant tenderness, or unexplained "flu like" symptoms). The manufacturer states that atomoxetine is contraindicated in patients currently receiving or having recently received (i.e., within 2 weeks) monoamine oxidase (MAO) inhibitor therapy. In addition, at least 2 weeks should elapse after discontinuing atomoxetine before initiating MAO inhibitor therapy. Severe, potentially fatal, reactions (including hyperthermia, rigidity, myoclonus, autonomic instability with possible rapid fluctuations of vital signs, and mental status changes that include extreme agitation progressing to delirium and coma) have been reported in patients receiving other drugs that affect brain monoamine concentrations concomitantly with MAO inhibitor therapy. For more Drug Warnings (Complete) data for ATOMOXETINE (26 total), please visit the HSDB record page. Pharmacodynamics Atomoxetine is a selective norepinephrine (NE) reuptake inhibitor used for the treatment of attention deficit hyperactivity disorder (ADHD). Atomoxetine has been shown to specifically increase norepinephrine and dopamine within the prefrontal cortex, which results in improved ADHD symptoms. Due to atomoxetine's noradrenergic activity, it also has effects on the cardiovascular system such as increased blood pressure and tachycardia. Sudden deaths, stroke, and myocardial infarction have been reported in patients taking atomoxetine at usual doses for ADHD. Atomoxetine should be used with caution in patients whose underlying medical conditions could be worsened by increases in blood pressure or heart rate such as certain patients with hypertension, tachycardia, or cardiovascular or cerebrovascular disease. It should not be used in patients with severe cardiac or vascular disorders whose condition would be expected to deteriorate if they experienced clinically important increases in blood pressure or heart rate. Although the role of atomoxetine in these cases is unknown, consideration should be given to not treating patients with clinically significant cardiac abnormalities. Patients who develop symptoms such as exertional chest pain, unexplained syncope, or other symptoms suggestive of cardiac disease during atomoxetine treatment should undergo a prompt cardiac evaluation. In general, particular care should be taken in treating ADHD in patients with comorbid bipolar disorder because of concern for possible induction of a mixed/manic episode in patients at risk for bipolar disorder. Treatment emergent psychotic or manic symptoms, e.g., hallucinations, delusional thinking, or mania in children and adolescents without a prior history of psychotic illness or mania can be caused by atomoxetine at usual doses. If such symptoms occur, consideration should be given to a possible causal role of atomoxetine, and discontinuation of treatment should be considered. Atomoxetine capsules increased the risk of suicidal ideation in short-term studies in children and adolescents with Attention-Deficit/Hyperactivity Disorder (ADHD). All pediatric patients being treated with atomoxetine should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases. Postmarketing reports indicate that atomoxetine can cause severe liver injury. Although no evidence of liver injury was detected in clinical trials of about 6000 patients, there have been rare cases of clinically significant liver injury that were considered probably or possibly related to atomoxetine use in postmarketing experience. Rare cases of liver failure have also been reported, including a case that resulted in a liver transplant. Atomoxetine should be discontinued in patients with jaundice or laboratory evidence of liver injury, and should not be restarted. Laboratory testing to determine liver enzyme levels should be done upon the first symptom or sign of liver dysfunction (e.g., pruritus, dark urine, jaundice, right upper quadrant tenderness, or unexplained “flu like” symptoms). |
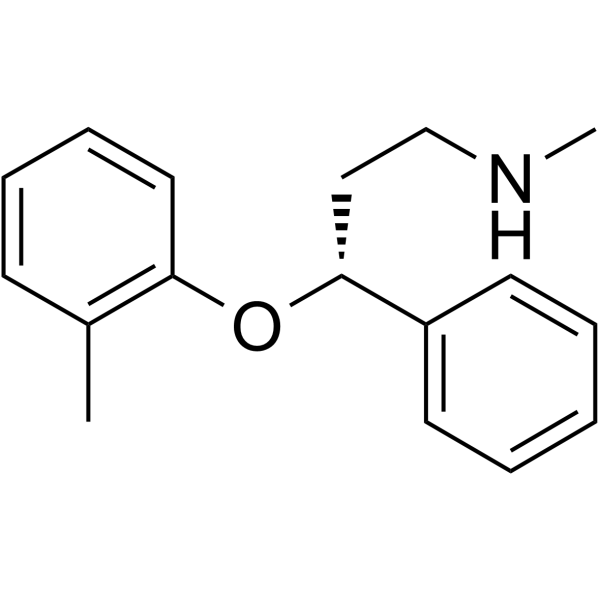
| 分子式 |
C17H21NO
|
|---|---|
| 分子量 |
255.354744672775
|
| 精确质量 |
255.162
|
| 元素分析 |
C, 79.96; H, 8.29; N, 5.49; O, 6.27
|
| CAS号 |
83015-26-3
|
| 相关CAS号 |
Atomoxetine hydrochloride;82248-59-7;(Rac)-Atomoxetine-d7 hydrochloride;Atomoxetine-d3 hydrochloride;1217776-38-9
|
| PubChem CID |
54841
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| 密度 |
1.0±0.1 g/cm3
|
| 沸点 |
389.0±37.0 °C at 760 mmHg
|
| 熔点 |
161-165 ºC
|
| 闪点 |
164.1±16.0 °C
|
| 蒸汽压 |
0.0±0.9 mmHg at 25°C
|
| 折射率 |
1.552
|
| LogP |
3.28
|
| tPSA |
21.26
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
2
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
19
|
| 分子复杂度/Complexity |
237
|
| 定义原子立体中心数目 |
1
|
| SMILES |
[C@H](C1C=CC=CC=1)(CCNC)OC1C=CC=CC=1C
|
| InChi Key |
VHGCDTVCOLNTBX-QGZVFWFLSA-N
|
| InChi Code |
InChI=1S/C17H21NO/c1-14-8-6-7-11-16(14)19-17(12-13-18-2)15-9-4-3-5-10-15/h3-11,17-18H,12-13H2,1-2H3/t17-/m1/s1
|
| 化学名 |
(3R)-N-methyl-3-(2-methylphenoxy)-3-phenylpropan-1-amine
|
| 别名 |
Atomoxetine; 83015-26-3; Tomoxetine; (-)-Tomoxetine; Tomoxetina; Tomoxetinum; Strattera; Tomoxetinum [Latin];
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.9162 mL | 19.5810 mL | 39.1619 mL | |
| 5 mM | 0.7832 mL | 3.9162 mL | 7.8324 mL | |
| 10 mM | 0.3916 mL | 1.9581 mL | 3.9162 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。