| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
Neuromuscular nicotinic acetylcholine receptor (nAChR) [1][5]
- Glioblastoma stem cell-related targets, astroglial differentiation-related markers (GFAP, Nestin) [4] - HLA-DRβ107:01 genotype-related inflammatory pathways [2] |
|---|---|
| 体外研究 (In Vitro) |
在 HSR040622 和 HSR040821 细胞中,苯磺酸阿曲库铵(10 µM;72 小时)刺激星形胶质细胞分化,但不刺激神经元分化 [4]。在体外进行 GSC 异种移植的小鼠中,苯磺酸阿曲库铵(10 µM;48 小时)可减少肿瘤移植并提高存活率 [4]。当暴露于苯磺酸阿曲库铵(2.4 µM;120 分钟)时,大鼠强直性收缩完全消失;大鼠趾长伸肌细胞的抽搐非常轻微[5]。
苯磺阿曲库铵(Atracurium Besylate, BW-33A)是一种非去极化型肌松药,可竞争性结合神经肌肉接头处的nAChR。在离体大鼠膈神经-膈肌标本中,它诱导剂量依赖性神经肌肉阻滞,浓度≥0.1 μM时出现强直衰减现象[5] - 在人胶质母细胞瘤细胞系(U87、U251、GB1)中,苯磺阿曲库铵(Atracurium Besylate, BW-33A)(10-100 μM)促进星形胶质细胞分化,表现为胶质纤维酸性蛋白(GFAP)表达升高和巢蛋白(Nestin,干细胞标志物)表达降低。它通过减少CD133+细胞数量和抑制球形成能力(50 μM时球形成率降低约60%)耗尽胶质母细胞瘤干细胞(GSCs)[4] - 苯磺阿曲库铵(Atracurium Besylate, BW-33A)以剂量依赖性方式抑制胶质母细胞瘤细胞增殖(U87细胞72小时IC50约75 μM),诱导G0/G1期细胞周期阻滞,浓度高达100 μM时对正常星形胶质细胞无明显细胞毒性[4] |
| 体内研究 (In Vivo) |
DBA/2 和 SJL 小鼠通过气管内注射苯磺酸盐(1、5、10、20、50 mg/kg)诱导支气管收缩[2]。经历神经肌肉抑制的大鼠接受苯磺酸气管注射(4.8 mg/kg)[3]。
在健康人体志愿者中,静脉注射苯磺阿曲库铵(Atracurium Besylate, BW-33A)(0.3-0.6 mg/kg)产生剂量依赖性神经肌肉阻滞。起效时间为2.5-4分钟,临床松弛持续时间(恢复至25%肌颤搐高度)为30-60分钟,完全恢复(100%肌颤搐高度)需60-90分钟,无残余肌 weakness[1] - 携带HLA-DRβ107:01基因型的患者中,静脉注射苯磺阿曲库铵(Atracurium Besylate, BW-33A)(0.5 mg/kg)诱发支气管收缩的风险是非携带者的3.2倍,表现为第1秒用力呼气容积(FEV1)降低和气道阻力升高[2] - 在四氯化碳诱导的炎症性肝病大鼠中,静脉注射苯磺阿曲库铵(Atracurium Besylate, BW-33A)(0.2 mg/kg)使神经肌肉阻滞持续时间较健康大鼠缩短约40%,该效应与肝脏代谢增强、药物清除加快相关[3] - 在U87胶质母细胞瘤异种移植裸鼠模型中,腹腔注射苯磺阿曲库铵(Atracurium Besylate, BW-33A)(20 mg/kg,每2天1次,持续3周)使肿瘤体积缩小约55%,肿瘤重量减轻约52%。它耗尽肿瘤组织中的GSCs(CD133+细胞比例降低约45%),促进星形胶质细胞分化(GFAP表达升高约2.3倍)[4] |
| 酶活实验 |
神经肌肉接头nAChR结合实验:将离体大鼠膈神经-膈肌标本置于器官浴中进行电刺激,加入0.05-1.0 μM 苯磺阿曲库铵(Atracurium Besylate, BW-33A),持续记录肌颤搐张力,绘制浓度-效应曲线评估神经肌肉阻滞效能[5]
- GSC球形成实验:胶质母细胞瘤细胞以低密度接种于无血清培养基,加入10-100 μM 苯磺阿曲库铵(Atracurium Besylate, BW-33A)处理7天后,显微镜下计数球的数量和大小,计算球形成效率[4] |
| 细胞实验 |
细胞增殖测定[4]
细胞类型:胶质母细胞瘤干细胞 (GSC) 测试浓度: 3、10、20 µM 孵育时间:72小时 实验结果:GFP阳性细胞的百分比以剂量依赖性方式从DMSO中的5.3%增加到15.4%, 3 μM、10 μM 和 20 μM 分别为 81.1% 和 86.8%。 胶质母细胞瘤细胞增殖实验:U87/U251/GB1细胞接种于96孔板,用0-200 μM 苯磺阿曲库铵(Atracurium Besylate, BW-33A)处理24-72小时,CCK-8法检测细胞活力,计算IC50值[4] - 星形胶质细胞分化实验:胶质母细胞瘤细胞用50 μM 苯磺阿曲库铵(Atracurium Besylate, BW-33A)处理5天,免疫荧光染色检测GFAP(星形胶质细胞标志物)和Nestin(干细胞标志物)表达,流式细胞术量化阳性细胞比例[4] - 细胞周期实验:U87细胞用75 μM 苯磺阿曲库铵(Atracurium Besylate, BW-33A)处理48小时,细胞经固定、碘化丙啶染色后,流式细胞术分析细胞周期分布[4] - 神经肌肉传递实验:离体大鼠膈肌与0.1-0.5 μM 苯磺阿曲库铵(Atracurium Besylate, BW-33A)孵育30分钟,记录诱发电位和肌颤搐反应,评估强直衰减和神经肌肉阻滞动力学[5] |
| 动物实验 |
Animal/Disease Models: 5-12 weeks, 15-20 g male mice [2]
Doses: 1, 5, 10, 20, 50 mg/kg Route of Administration: intravenous (iv) (iv)injection Experimental Results: Induced bronchoconstriction and Atracurium-induced airway hyperresponsiveness was abolished in a dose-dependent manner by atropine or pancuronium pretreatment. Animal/Disease Models: 290 ± 30 g male Sprague ± Dawley rats (60 mg/kg heat-killed Corynebacterium parvum intravenously (iv) (iv)(iv)) [3] Doses: 4.8 mg/kg Route of Administration: intravenous (iv) (iv)injection Experimental Results: In large mice injected with Corynebacterium parvum Induction of neuromuscular blockade in rats. Healthy human volunteer study (clinical in vivo): Volunteers received intravenous Atracurium Besylate (BW-33A) at doses of 0.3, 0.45, or 0.6 mg/kg. Neuromuscular function was monitored by train-of-four (TOF) stimulation of the ulnar nerve, with twitch height recorded continuously until full recovery [1] - Inflammatory liver disease rat model: Rats were treated with carbon tetrachloride to induce chronic liver inflammation. After 8 weeks, Atracurium Besylate (BW-33A) (0.2 mg/kg) was administered intravenously. Neuromuscular blockade duration was measured by TOF stimulation of the sciatic nerve, with recovery time to 25% twitch height recorded [3] - Glioblastoma xenograft model: Nude mice were subcutaneously inoculated with U87 glioblastoma cells. When tumors reached ~150 mm³, mice were randomized into control and treatment groups. Atracurium Besylate (BW-33A) was dissolved in normal saline and administered intraperitoneally at 20 mg/kg once every 2 days for 3 weeks. Tumor volume was measured every 3 days; mice were sacrificed to collect tumors for immunohistochemical and flow cytometry analysis [4] - Bronchoconstriction study: Patients undergoing surgery were genotyped for HLA-DRβ107:01. Those receiving Atracurium Besylate (BW-33A) (0.5 mg/kg, intravenous) were monitored for airway resistance and FEV1 within 30 minutes of administration [2] |
| 药代性质 (ADME/PK) |
Biological Half-Life
The elimination half-life is approximately 20 minutes. In healthy humans, Atracurium Besylate (BW-33A) has a volume of distribution of ~0.2 L/kg and a plasma clearance of ~5.5 mL/kg/min. The elimination half-life (t1/2β) is ~20 minutes [1] - It undergoes Hoffman elimination (pH- and temperature-dependent) and ester hydrolysis in plasma, with minimal reliance on hepatic or renal function for clearance in healthy individuals [1] - In rats with inflammatory liver disease, plasma clearance of Atracurium Besylate (BW-33A) increased by ~35%, and elimination half-life shortened to ~12 minutes [3] - Plasma protein binding is ~82%, primarily to albumin [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation No information is available on the use of atracurium during breastfeeding. Because it is short acting, highly polar and poorly absorbed orally, it is not likely to reach the breastmilk in high concentration or to reach the bloodstream of the infant. When a combination of anesthetic agents is used for a procedure, follow the recommendations for the most problematic medication used during the procedure. Consider using an atracurium product that has no benzyl alcohol preservative. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Acute toxicity: The median lethal dose (LD50) in mice is ~2.5 mg/kg (intravenous) [1] - Bronchoconstriction: In patients carrying the HLA-DRβ107:01 genotype, Atracurium Besylate (BW-33A) may induce dose-dependent bronchoconstriction, a hypersensitivity-related side effect [2] - No significanttoxicity was observed in healthy humans or animals at clinical/research doses [1][3][4] |
| 参考文献 |
|
| 其他信息 |
Atracurium besylate is the bisbenzenesulfonate salt of atracurium. It has a role as a nicotinic antagonist and a muscle relaxant. It is a quaternary ammonium salt and an organosulfonate salt. It contains an atracurium.
A non-depolarizing neuromuscular blocking agent with short duration of action. Its lack of significant cardiovascular effects and its lack of dependence on good kidney function for elimination provide clinical advantage over alternate non-depolarizing neuromuscular blocking agents. Atracurium Besylate is a synthetic dibenzensulfonate derivative muscle relaxant, Atracurium Besylate acts as a non-depolarizing neuromuscular blocking agent, with short to intermediary duration of action and no significant cardiovascular effects. Not dependent on kidney function for elimination, it provides clinical advantages over other non-depolarizing, neuromuscular blocking agents. (NCI04) A non-depolarizing neuromuscular blocking agent with short duration of action. Its lack of significant cardiovascular effects and its lack of dependence on good kidney function for elimination provide clinical advantage over alternate non-depolarizing neuromuscular blocking agents. See also: Atracurium (has active moiety). Drug Indication For use, as an adjunct to general anesthesia, to facilitate endotracheal intubation and to provide skeletal muscle relaxation during surgery or mechanical ventilation. Mechanism of Action Atracurium antagonizes the neurotransmitter action of acetylcholine by binding competitively with cholinergic receptor sites on the motor end-plate. This antagonism is inhibited, and neuromuscular block reversed, by acetylcholinesterase inhibitors such as neostigmine, edrophonium, and pyridostigmine. Pharmacodynamics Atracurium is a nondepolarizing skeletal muscle relaxant. Atracurium can be used most advantageously if muscle twitch response to peripheral nerve stimulation is monitored to assess degree of muscle relaxation. The duration of neuromuscular block produced by Atracurium is approximately one third to one half the duration of block by d-tubocurarine, metocurine, and pancuronium at initially equipotent doses. As with other nondepolarizing neuromuscular blockers, the time to onset of paralysis decreases and the duration of maximum effect increases with increasing doses of Atracurium. Repeated administration of maintenance doses of Atracurium has no cumulative effect on the duration of neuromuscular block if recovery is allowed to begin prior to repeat dosing. Moreover, the time needed to recover from repeat doses does not change with additional doses. Repeat doses can therefore be administered at relatively regular intervals with predictable results. Atracurium Besylate (BW-33A) is a non-depolarizing neuromuscular blocking agent clinically used to induce muscle relaxation during anesthesia and mechanical ventilation [1] - Its neuromuscular blocking mechanism involves competitive antagonism of nAChR at the neuromuscular junction, preventing acetylcholine binding and muscle contraction [5] - Beyond its clinical use, it exhibits potential antitumor activity against glioblastoma by promoting astroglial differentiation and depleting GSCs, expanding its pharmacological profile [4] - Hoffman elimination ensures predictable clearance even in patients with impaired hepatic or renal function, reducing the risk of residual neuromuscular blockade [1] |
| 分子式 |
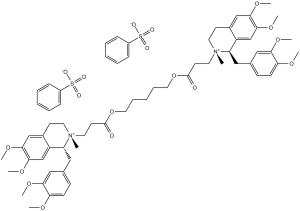
C53H72N2O12.2(C6H5O3S)
|
|
|---|---|---|
| 分子量 |
1243.48
|
|
| 精确质量 |
1242.5
|
|
| CAS号 |
64228-81-5
|
|
| 相关CAS号 |
Atracurium;64228-79-1
|
|
| PubChem CID |
47320
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 熔点 |
85-90ºC
|
|
| LogP |
11.326
|
|
| tPSA |
257.6
|
|
| 氢键供体(HBD)数目 |
0
|
|
| 氢键受体(HBA)数目 |
18
|
|
| 可旋转键数目(RBC) |
26
|
|
| 重原子数目 |
87
|
|
| 分子复杂度/Complexity |
1560
|
|
| 定义原子立体中心数目 |
0
|
|
| InChi Key |
XXZSQOVSEBAPGS-UHFFFAOYSA-L
|
|
| InChi Code |
InChI=1S/C53H72N2O12.2C6H6O3S/c1-54(22-18-38-32-48(62-7)50(64-9)34-40(38)42(54)28-36-14-16-44(58-3)46(30-36)60-5)24-20-52(56)66-26-12-11-13-27-67-53(57)21-25-55(2)23-19-39-33-49(63-8)51(65-10)35-41(39)43(55)29-37-15-17-45(59-4)47(31-37)61-6;2*7-10(8,9)6-4-2-1-3-5-6/h14-17,30-35,42-43H,11-13,18-29H2,1-10H3;2*1-5H,(H,7,8,9)/q+2;;/p-2
|
|
| 化学名 |
benzenesulfonate;5-[3-[1-[(3,4-dimethoxyphenyl)methyl]-6,7-dimethoxy-2-methyl-3,4-dihydro-1H-isoquinolin-2-ium-2-yl]propanoyloxy]pentyl 3-[1-[(3,4-dimethoxyphenyl)methyl]-6,7-dimethoxy-2-methyl-3,4-dihydro-1H-isoquinolin-2-ium-2-yl]propanoate
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (2.01 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (2.01 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (2.01 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 100 mg/mL (80.42 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.8042 mL | 4.0210 mL | 8.0419 mL | |
| 5 mM | 0.1608 mL | 0.8042 mL | 1.6084 mL | |
| 10 mM | 0.0804 mL | 0.4021 mL | 0.8042 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。