| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
| 靶点 |
PIM2 kinase
Pan-Pim kinases (Pim-1, Pim-2, Pim-3), serine/threonine kinases. For AZD1208, the IC50 values were: Pim-1 = 0.4 nM, Pim-2 = 2.0 nM, Pim-3 = 0.6 nM (measured via HTRF kinase assay). It exhibited high selectivity over 45 other kinases (e.g., Src, Akt, ERK) with IC50 > 10 μM, confirming pan-Pim specificity [1] |
|---|---|
| 体外研究 (In Vitro) |
在巨核细胞白血病细胞系 MOLM-16 中,AZD1208 表现出强大的抗增殖活性,GI50 值低于 100 nM 就证明了这一点 [1]。 AZD1208 (10 μM) 在 1 μM 浓度下显着抑制所有细胞中的 PIM 激酶,在 1 μM 浓度下抑制 Ramos 细胞增殖。 PIM2 敲低主要与细胞周期的改变有关,而 AZD1208 会导致细胞凋亡 [2]。当 AZD1208 和 AZD2014 组合时,AKT 和 4EBP1 激活被显着抑制,多核糖体形成被抑制,并且 AMPKα(翻译机器的负调节因子)通过 AML 细胞中的 mTORC1/2 信号传导快速激活 [3]。
酶活与增殖抑制:AZD1208(0.001 nM–10 μM)可剂量依赖性抑制重组Pim-1/2/3:在0.4 nM(Pim-1)、2.0 nM(Pim-2)、0.6 nM(Pim-3)时实现50%抑制 [1]。在人癌细胞系(前列腺癌:DU145、LNCaP;乳腺癌:MDA-MB-231)中,通过MTT法检测其抑制增殖的IC50分别为5.2 μM(DU145)、7.8 μM(LNCaP)、9.5 μM(MDA-MB-231);Western blot显示DU145细胞在5 μM时p-Bad(Ser112位点)减少60% [1] - 非霍奇金淋巴瘤(NHL)细胞:在Pim-2正常(SU-DHL-4)和Pim-2缺失(SU-DHL-4 shPim2)的NHL细胞中,AZD1208(1 μM–10 μM)处理72小时后,Pim-2缺失细胞的抗增殖效应增强:IC50=2.5 μM(shPim2) vs. 5.8 μM(对照)。流式细胞术显示,shPim2细胞+5 μM AZD1208的凋亡率达42%(对照+5 μM AZD1208为20%),切割型caspase-3增加3.0倍 [2] - AML细胞:在AML细胞系(MV4-11、MOLM-13)中,AZD1208(2 μM)联合AZD2014(1 μM)通过CCK-8法检测,细胞活力降低75%(单独AZD1208为35%,单独AZD2014为25%)。Western blot显示HSF1减少50%、HSP70减少60%;qRT-PCR证实HSF靶基因(HSP90)下调45% [3] |
| 体内研究 (In Vivo) |
AZD1208 以剂量成比例的方式抑制体内 MOLM-16 和 KG-1a 异种移植肿瘤的生长。
与对照组相比,患病(NZB × NZW)F1小鼠的肾溶物、SLE患者的pbmc和LN患者的肾活检组织中Pim-1表达上调(均P < 0.05)。Pim-1抑制剂AZD1208降低患病(NZB × NZW)F1小鼠蛋白尿、肾小球肾炎、肾免疫复合物沉积的严重程度和血清抗dsdna抗体水平,同时抑制NFATc1表达和NLRP3炎性体激活(与对照组相比,各P < 0.05)。此外,在小鼠和人足细胞中,靶向小干扰RNA (siRNA)敲低Pim-1在抗ddna阳性血清存在下抑制NFATc1和NLRP3炎症小体信号传导(与对照siRNA相比,P < 0.05)。在机制上,Pim-1通过细胞内Ca2+调节NLRP3炎性体的激活(与正常对照组相比P < 0.05)。阻断Pim-1的治疗效果在MRL/lpr小鼠中得到了重复。 结论:这些数据表明Pim-1是SLE患者LN发病的关键调节因子。因此,靶向Pim-1/NFATc1/NLRP3通路可能具有治疗人类LN的潜力。Reference: Arthritis Rheumatol. 2019 Aug;71(8):1308-1318. https://onlinelibrary.wiley.com/doi/abs/10.1002/art.40863 NHL异种移植模型:6周龄雌性裸鼠接种SU-DHL-4细胞构建异种移植模型,随机分为3组(每组n=6):溶媒组(0.5%甲基纤维素)、AZD1208 50 mg/kg组、AZD1208 100 mg/kg组。药物口服给药,每日一次,连续21天。肿瘤体积较溶媒组减少40%(50 mg/kg)和65%(100 mg/kg);肿瘤重量减少35%(50 mg/kg)和60%(100 mg/kg)。免疫组化显示Ki-67(增殖标志物)在100 mg/kg时减少55% [2] - AML小鼠模型:8周龄雄性NOD/SCID小鼠注射MV4-11细胞,随机分为4组(每组n=8):溶媒组、AZD1208 50 mg/kg组、AZD2014 20 mg/kg组、联合组。AZD1208口服每日一次,AZD2014口服每日两次,连续14天。联合组生存期延长50%(单独AZD1208为20%,单独AZD2014为15%),骨髓白血病原始细胞比例从溶媒组的85%降至30% [3] |
| 酶活实验 |
HTRF法Pim激酶抑制实验:将重组人Pim-1(44–313位氨基酸)、Pim-2(38–326位氨基酸)或Pim-3(41–323位氨基酸)与生物素化肽底物(Pim-1/3用RRRVSYRRR,Pim-2用RRRLSYRRR,20 μM)、Eu标记抗磷酸肽抗体及[γ-³³P]-ATP(10 μM)共同孵育于激酶缓冲液(25 mM Tris-HCl pH 7.5、10 mM MgCl₂、1 mM DTT)中。加入系列稀释的AZD1208(0.001 nM–10 μM),30°C孵育60分钟。检测时间分辨荧光(激发光340 nm,发射光620 nm),通过四参数逻辑回归计算IC50 [1]
|
| 细胞实验 |
流式细胞术检测细胞内磷酸化蛋白的表达水平[3]
用AZD1208 (2 μM)、AZD2014 (1 μM)或两者联合处理MOLM-16和OCI-AML3细胞6 h。然后用1.6%多聚甲醛固定细胞,并在冷冻甲醇(70% PBS;1 mL/百万细胞),静置20分钟。洗涤两次后,细胞在1%牛血清白蛋白PBS中重悬。将抗体加入细胞悬液中孵育30 min。所用抗体为Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (E10)小鼠单克隆抗体;兔Phospho-S6核糖体蛋白(Ser235/236) (D57.2.2E) XP单克隆抗体;Phospho-Akt (Ser473)兔单克隆抗体;和趋化因子受体CXCR4。洗涤两次后,细胞重悬,用Gallios流式细胞仪分析。 单独分析[3] 在Methocult H4435中以0.05-0.1×106 cells/mL的浓度接种单个核细胞。电镀前在培养基中加入AZD1208 (1 μM或3 μM)、AZD2014 (0.25 μM或0.5 μM)或两者的组合。将细胞置于含有2×2-mm网格(NUNC)的35×10-mm培养皿中,一式三份,37°C, 5% CO2,加湿室中孵育14天。利用1×-3立体镜对菌落进行评分。 Polysomal试验[3] 分别用2 μM、3 μM AZD1208、1 μM AZD2014或两者联合作用Molm-16和OCI-AML3细胞6 h。然后用添加环己亚胺(100 μg/mL)的PBS洗涤细胞2次,重悬于低渗裂解缓冲液(5 mM Tris, pH 7.5;2.5 mM MgCl2;1.5 mM KCl),添加环己亚胺(100 μg/mL)、二硫苏糖醇(2 mM)、蛋白酶抑制剂、RNase抑制剂(1 U/μL)。将悬浮液涡旋4 s后,加入Triton ×100(0.5%)和脱氧胆酸钠(0.5%)。在4°C下,以12,000g旋转5分钟后,将上清液转移到新管中,在液氮中快速冷冻。如前所述进行多体分离。 癌细胞增殖实验:将DU145/LNCaP细胞以5×10³个细胞/孔接种于96孔板,用AZD1208(0.1 μM–20 μM)处理72小时。加入5 mg/mL MTT试剂孵育4小时,DMSO溶解甲臜结晶后,检测570 nm处吸光度,计算IC50 [1] - NHL细胞凋亡实验:将SU-DHL-4(shPim2/对照)细胞以2×10⁵个细胞/孔接种于6孔板,用5 μM AZD1208处理48小时。Annexin V-FITC/PI染色后流式细胞术分析凋亡;Western blot检测切割型caspase-3和p-Bad [2] - AML细胞HSF通路实验:将MV4-11细胞以3×10⁵个细胞/孔接种于6孔板,用AZD1208(2 μM)+AZD2014(1 μM)处理24小时。裂解细胞后,Western blot检测HSF1/HSP70;提取总RNA,qRT-PCR定量HSP90 mRNA [3] |
| 动物实验 |
Dissolved in 0.5% hydroxypropyl methylcellulose; 30 mg/kg twice per week; oral gavage Female CB17 SCID mice implanted with MOLM-16 cells (5 × 106) or KG-1a cells (6 × 106)
Treatment protocols: Female (NZB × NZW)F1 mice were orally treated with AZD1208 (15 mg/kg) or vehicle control (0.1% Tween 80 and 0.5% methyl cellulose in water) 19, 20 for 12 weeks (n = 10 mice per group), starting at age 22 weeks (at the time of onset of proteinuria). Mice were then placed under anesthesia and killed at age 34 weeks.
Twelve-week-old MRL/lpr mice received the selective Pim-1 inhibitor SMI-4a (60 mg/kg) or vehicle control (DMSO/PEG-400/Tween 80) twice daily, as described previously 21. Oral gavage was administered on 5 of 7 days each week for 8 weeks (n = 10 mice per group). In an independent experiment, survival was observed in mice until age 30 weeks, and the survival rates were compared between 2 groups (n = 15 mice per group). Reference: Arthritis Rheumatol. 2019 Aug;71(8):1308-1318. https://onlinelibrary.wiley.com/doi/abs/10.1002/art.40863
NHL Xenograft Protocol: Female nude mice were subcutaneously injected with 5×10⁶ SU-DHL-4 cells. When tumors reached ~100 mm³, mice were grouped. AZD1208 was dissolved in 0.5% methylcellulose and administered orally once daily for 21 days. Tumor volume (length×width²/2) was measured every 3 days; mice were euthanized on day 21, and tumors were weighed/processed for immunohistochemistry [2] - AML Mouse Protocol: Male NOD/SCID mice were intravenously injected with 1×10⁶ MV4-11 cells. After 7 days, AZD1208 (50 mg/kg, oral, once daily) and AZD2014 (20 mg/kg, oral, twice daily) were administered for 14 days. Survival was monitored daily; bone marrow was collected at euthanasia to count leukemic blasts [3] |
| 药代性质 (ADME/PK) |
In male C57BL/6 mice, oral AZD1208 (50 mg/kg) had an oral bioavailability of 35%, maximum plasma concentration (Cmax) of 2.8 μM, time to Cmax (Tmax) of 1.8 hours, and terminal half-life (t₁/₂) of 4.2 hours [1]
- Intravenous AZD1208 (10 mg/kg) in mice showed clearance (CL) of 12 mL/min/kg and steady-state volume of distribution (Vss) of 0.9 L/kg [1] - Human plasma protein binding of AZD1208 was 97% via equilibrium dialysis [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
In NHL xenograft mice (AZD1208 100 mg/kg, 21 days), no significant weight loss or clinical toxicity (lethargy, diarrhea) was observed. Serum ALT/AST/creatinine were normal, and liver/kidney histology showed no abnormalities [2]
- In AML mice (combination treatment), AZD1208 (50 mg/kg) caused mild thrombocytopenia (platelet count reduced by 15%) but no other hematological or organ toxicity [3] - In human normal peripheral blood mononuclear cells (PBMCs), AZD1208 (up to 20 μM) showed <10% cytotoxicity, indicating cancer cell selectivity [1] |
| 参考文献 |
|
| 其他信息 |
pan-PIM Kinase Inhibitor AZD1208 is an orally available, small molecule inhibitor of PIM kinases with potential antineoplastic activity. Pan-PIM kinase inhibitor AZD1208 inhibits the activities of PIM1, PIM2 and PIM3 serine/threonine kinases, which may result in the interruption of the G1/S phase cell cycle transition, thereby causing cell cycle arrest and inducing apoptosis in cells that overexpress PIMs. The growth inhibition of several leukemia cell lines by this agent is correlated with the expression levels of PIM1, which is the substrate of STAT transcription factors. PIM kinases are downstream effectors of many cytokine and growth factor signaling pathways and are upregulated in various malignancies.
AZD1208 is a potent, oral pan-Pim kinase inhibitor developed for hematological malignancies (NHL, AML) and solid tumors (prostate, breast cancer) [1][2][3] - Its mechanism involves inhibiting Pim-mediated phosphorylation of substrates (p-Bad, p-c-Myc) to induce apoptosis, and synergizing with mTOR inhibitors (AZD2014) in AML by suppressing the HSF pathway (reducing HSF1/HSPs) [2][3] - Pim-2 loss enhances AZD1208’s efficacy in NHL, suggesting Pim-2 expression as a potential predictive biomarker for treatment response [2] |
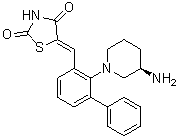
| 分子式 |
C21H21N3O2S
|
|
|---|---|---|
| 分子量 |
379.48
|
|
| 精确质量 |
379.135
|
|
| 元素分析 |
C, 66.47; H, 5.58; N, 11.07; O, 8.43; S, 8.45
|
|
| CAS号 |
1204144-28-4
|
|
| 相关CAS号 |
AZD1208 hydrochloride;1621866-96-3
|
|
| PubChem CID |
58423153
|
|
| 外观&性状 |
Light yellow to yellow solid powder
|
|
| 密度 |
1.3±0.1 g/cm3
|
|
| 折射率 |
1.677
|
|
| LogP |
2.38
|
|
| tPSA |
100.73
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
5
|
|
| 可旋转键数目(RBC) |
3
|
|
| 重原子数目 |
27
|
|
| 分子复杂度/Complexity |
602
|
|
| 定义原子立体中心数目 |
1
|
|
| SMILES |
C1C[C@H](CN(C1)C2=C(C=CC=C2C3=CC=CC=C3)/C=C\4/C(=O)NC(=O)S4)N
|
|
| InChi Key |
MCUJKPPARUPFJM-UWCCDQBKSA-N
|
|
| InChi Code |
InChI=1S/C21H21N3O2S/c22-16-9-5-11-24(13-16)19-15(12-18-20(25)23-21(26)27-18)8-4-10-17(19)14-6-2-1-3-7-14/h1-4,6-8,10,12,16H,5,9,11,13,22H2,(H,23,25,26)/b18-12-/t16-/m1/s1
|
|
| 化学名 |
(5Z)-5-[[2-[(3R)-3-aminopiperidin-1-yl]-3-phenylphenyl]methylidene]-1,3-thiazolidine-2,4-dione
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 5 mg/mL (13.18 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 50.0 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 5 mg/mL (13.18 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 50.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (6.59 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6352 mL | 13.1759 mL | 26.3518 mL | |
| 5 mM | 0.5270 mL | 2.6352 mL | 5.2704 mL | |
| 10 mM | 0.2635 mL | 1.3176 mL | 2.6352 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT01489722 | Terminated | Drug: AZD1208 | Acute Myeloid Leukemia | AstraZeneca | February 2012 | Phase 1 |
| NCT01588548 | Completed Has Results | Drug: AZD1208 | Advanced Solid Malignancies Malignant Lymphoma |
AstraZeneca | July 2012 | Phase 1 |