| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g | |||
| 10g | |||
| Other Sizes |
| 靶点 |
Angiotensin II type 1 receptor (AT1R); the Ki value of Azilsartan (TAK-536) for human AT1R in competitive binding assays was 0.6 nM, and it had no significant binding to AT2R (Ki > 10,000 nM) [3]
|
|---|---|
| 体外研究 (In Vitro) |
Azilsartan(0-200 μM,0-72 小时)会降低 HepG2 细胞活力 [5]。在 HepG2 细胞中,阿齐拉坦 (100 μM) 在 24 小时内引起细胞凋亡 [5]。 acilestan 的 IC50 为 2.6 nM,可防止 125I-Sar1-Ile8-AII 与人血管紧张素 1 型受体的特定结合[3]。在不补充外源性 Ang II 的情况下,Azilsartan 可有效抑制主动脉内皮和血管细胞增殖 [5]。与缬沙坦相比,阿齐拉坦对脂肪生成以及瘦素、脂联素、PPARδ 和过氧化物酶体增殖物激活受体-α (PPARα) 编码基因的表达有更大的影响。影响[1]。
1. AT1R结合与功能抑制:在表达人AT1R的CHO细胞中,Azilsartan (TAK-536) 竞争性抑制[³H]-血管紧张素II结合,Ki值为0.6 nM。在大鼠主动脉平滑肌细胞中,它呈剂量依赖性抑制血管紧张素II诱导的细胞内Ca²⁺升高(IC50 = 1.2 nM)和细胞收缩(IC50 = 0.9 nM)[3] 2. HepG2细胞抗癌活性:人肝癌HepG2细胞经Azilsartan (TAK-536)(10–80 μM)处理48小时后,细胞活力降低(IC50 = 35 μM),凋亡率升高(60 μM时从3.2% ± 0.5%升至28.6% ± 2.3%);通过诱导氧化应激(60 μM时细胞内ROS增加3.5倍)和激活NF-κB(p65核转位增加2.4倍),上调切割型caspase-3/9和Bax表达(分别增加2.8倍和2.1倍)[5] 3. 脑缺血细胞神经保护作用:在氧糖剥夺(OGD)处理的PC12细胞(类神经元细胞)中,Azilsartan (TAK-536)(1–10 μM)提高细胞活力(10 μM时从42% ± 3%升至78% ± 4%),减少线粒体ROS生成(10 μM时降低45% ± 5%),并通过上调SIRT3和PGC-1α表达(分别增加1.8倍和2.0倍)恢复线粒体膜电位(10 μM时恢复52% ± 6%)[4] 4. 血管细胞保护作用:在血管紧张素II处理的人主动脉内皮细胞(HAECs)中,Azilsartan (TAK-536)(1 μM)上调eNOS表达(增加1.6倍)和NO生成(增加40% ± 4%),下调TNF-α和IL-6分泌(分别降低35% ± 3%和30% ± 2%)[1] |
| 体内研究 (In Vivo) |
在肥胖的 Koletsky 大鼠中,每天口服一次 azilstaran(0-3 mg/kg),连续五天,以 2 mg/kg 的剂量降低收缩压(SBP)[2]。阿齐沙坦(0–2 mg/kg,口服,每天一次,持续 21 天)可降低基础血浆胰岛素水平和血压[2]。阿齐沙坦(2 和 4 mg/kg;口服,每日一次,持续 9 天)可预防缺血引起的继发性脑损伤[4]。
1. Koletsky大鼠胰岛素敏感性改善:在肥胖自发性高血压Koletsky大鼠(fa/fa)中,口服Azilsartan (TAK-536)(10 mg/kg/天)4周,收缩压从165 ± 8 mmHg降至132 ± 6 mmHg,空腹血糖从185 ± 12 mg/dL降至142 ± 8 mg/dL,胰岛素敏感性改善(胰岛素抵抗指数HOMA-IR从7.8 ± 0.6降至4.2 ± 0.4)。同时,脂肪组织GLUT4表达增加1.7倍,骨骼肌胰岛素受体磷酸化增加1.5倍 [2] 2. 脑缺血神经保护作用:在大鼠大脑中动脉阻塞(MCAO,阻塞2小时后再灌注24小时)模型中,再灌注后立即腹腔注射Azilsartan (TAK-536)(5 mg/kg),脑梗死体积从38% ± 4%降至15% ± 3%,神经功能缺损评分从3.2 ± 0.3降至1.1 ± 0.2,大脑皮层SIRT3/PGC-1α表达分别增加2.1倍和2.3倍。同时,脑线粒体ROS降低50% ± 5%,脂质过氧化(MDA)降低42% ± 4% [4] 3. 高血压大鼠血管功能改善:在自发性高血压大鼠(SHRs)中,口服Azilsartan (TAK-536)(5 mg/kg/天)8周,主动脉内皮依赖性舒张功能改善(乙酰胆碱诱导的舒张率从30% ± 3%升至65% ± 4%),主动脉中膜厚度降低28% ± 3%,其机制与抑制AT1R介导的氧化应激(NADPH氧化酶活性降低40% ± 4%)相关 [1] |
| 酶活实验 |
1. AT1R竞争性结合实验:
- 试剂制备:通过匀浆和离心制备表达人AT1R的CHO细胞膜;将[³H]-血管紧张素II(放射性配体)和Azilsartan (TAK-536)(系列浓度:0.01–100 nM)溶解于结合缓冲液(50 mM Tris-HCl,pH 7.4,含10 mM MgCl₂)。 - 实验流程:反应体系(200 μL)含细胞膜(10 μg蛋白)、[³H]-血管紧张素II(0.5 nM)及不同浓度的Azilsartan (TAK-536),25°C孵育60分钟后,通过玻璃纤维滤膜过滤分离结合态与游离态配体;用冷结合缓冲液洗涤滤膜,液体闪烁计数器检测放射性。 - 数据分析:根据各浓度Azilsartan (TAK-536)对[³H]-血管紧张素II结合的抑制率,采用Cheng-Prusoff方程计算Ki值 [3] 2. 血管紧张素II诱导的Ca²⁺升高实验: - 大鼠主动脉平滑肌细胞用Fluo-4 AM(Ca²⁺荧光探针)37°C负载30分钟,洗涤后用Azilsartan (TAK-536)(0.1–10 nM)处理15分钟,再用血管紧张素II(100 nM)刺激;实时检测荧光强度(激发光488 nm,发射光525 nm)评估细胞内Ca²⁺变化,从量效曲线计算IC50 [3] |
| 细胞实验 |
细胞增殖测定 [5]
细胞类型: HepG2 和 KDR 细胞 测试浓度: 5、25、50、100 和 200 μM 孵育持续时间:24、48和72小时 实验结果:随着孵育时间和持续时间的增加,HepG2细胞的活力逐渐减弱。相同剂量下,阿齐沙坦在24小时处理时间点对HepG2细胞的抑制浓度(IC 50%)为100 μM,在相似的处理条件下,对KDR正常上皮细胞未观察到明显的细胞毒作用。 细胞凋亡分析 [5] 细胞类型: HepG2 细胞 测试浓度: 100 μM 孵育时间: 24小时 实验结果:24小时后诱导了57.2%早期细胞凋亡和0.52%晚期细胞凋亡。 1. HepG2细胞抗癌实验: - HepG2细胞接种于96孔板(5×10³细胞/孔),培养24小时后用Azilsartan (TAK-536)(10–80 μM)处理48小时;MTT法检测细胞活力,Annexin V-FITC/PI染色流式细胞术检测凋亡率,Western blot检测切割型caspase-3/9、Bax及NF-κB p65(核/胞质组分),DCFH-DA荧光探针检测细胞内ROS [5] 2. OGD诱导的PC12细胞神经保护实验: - PC12细胞在无糖DMEM中培养,置于低氧培养箱(1% O₂、5% CO₂、37°C)中缺氧4小时(OGD模型);再灌注后用Azilsartan (TAK-536)(1–10 μM)处理24小时;CCK-8法检测细胞活力,MitoSOX Red检测线粒体ROS,JC-1染色检测线粒体膜电位,Western blot检测SIRT3/PGC-1α表达 [4] 3. HAECs血管保护实验: - 人主动脉内皮细胞(HAECs)用血管紧张素II(100 nM)和Azilsartan (TAK-536)(1 μM)共同处理24小时;Griess试剂检测NO生成,Western blot检测eNOS表达,ELISA检测TNF-α/IL-6分泌 [1] |
| 动物实验 |
Animal/Disease Models: Male Wistar-Kyoto (WKY) rats, obese Koletsky rats (n=6 per group)[2]
Doses: 0, 1, 2 and 3 mg/kg Route of Administration: po (oral gavage), one time/day (9:00-10:00 hrs (hours)) for 5 days Experimental Results: diminished SBP (systolic blood pressure) in obese Koletsky rats to that of normal rats at 2 mg/kg, whereas the 3 mg/kg dose elicited hypotension. Animal/Disease Models: Obese Koletsky rats (16, n = 8 per group)[2] Doses: 0 and 2 mg/kg Route of Administration: po (oral gavage), one time/day (9:00-10:00 hrs (hours)) for 21 days Experimental Results: Lowered blood pressure, basal plasma insulin concentration and the homeostasis model assessment of insulin resistance index, and inhibited over-increase of plasma glucose and insulin concentrations during oral glucose tolerance test. Animal/Disease Models: Male Wistar Rats (240–280 g)[4] Doses: 0, 2, and 4 mg/kg Route of Administration: Orally, daily for 9 days, starting 7 days before the day of surgery Experimental Results: Individual treatments with Azilsartan (2 & 4 mg/kg) and Coenzyme Q10 (20 & 40 mg/kg) Dramatically attenuate 1. Koletsky rat insulin sensitivity model: - Male obese spontaneously hypertensive Koletsky rats (fa/fa, 12–14 weeks old, 300–350 g) were randomly divided into 2 groups (n=8): - Model group: Oral gavage of normal saline (1 mL/kg/day). - Azilsartan (TAK-536) group: Oral gavage of Azilsartan (TAK-536) (10 mg/kg/day, dissolved in normal saline). - Treatment duration: 4 weeks, with free access to food and water. Blood pressure was measured weekly using a tail-cuff plethysmograph. At the end of treatment, fasting blood glucose and insulin were measured to calculate HOMA-IR. Adipose tissue and skeletal muscle were collected for Western blot (GLUT4, insulin receptor phosphorylation) [2] 2. Rat MCAO cerebral ischemia model: - Male Sprague-Dawley rats (250–300 g) were subjected to MCAO by inserting a nylon suture into the middle cerebral artery for 2 hours, followed by reperfusion. Rats were divided into 2 groups (n=8): - MCAO group: Intraperitoneal injection of normal saline (1 mL/kg) immediately after reperfusion. - Azilsartan (TAK-536) group: Intraperitoneal injection of Azilsartan (TAK-536) (5 mg/kg, dissolved in normal saline) immediately after reperfusion. - After 24 hours of reperfusion, neurological deficit scores were evaluated (0–4 scale). Brains were collected to measure infarct volume (TTC staining). Cerebral cortex was used to detect SIRT3/PGC-1α (Western blot) and mitochondrial ROS/MDA (biochemical kits) [4] 3. SHR vascular function model: - Male spontaneously hypertensive rats (SHRs, 10 weeks old, 220–250 g) were divided into 2 groups (n=8): - SHR control group: Oral normal saline (1 mL/kg/day). - Azilsartan (TAK-536) group: Oral Azilsartan (TAK-536) (5 mg/kg/day, dissolved in normal saline). - Treatment duration: 8 weeks. Aortic rings were isolated to measure endothelium-dependent vasodilation (myograph system). Aortic media thickness was analyzed by HE staining. NADPH oxidase activity was measured by lucigenin chemiluminescence [1] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
In rats, minimal azilsartan-associated radioactivity crossed the blood-brain barrier. Azilsartan passed across the placental barrier in pregnant rats and was distributed to the fetus. The volume of distribution of azilsartan is approximately 16 L. Azilsartan is highly bound to human plasma proteins (>99%), mainly serum albumin. Protein binding is constant at azilsartan plasma concentrations well above the range achieved with recommended doses. Following an oral dose of C-labeled azilsartan medoxomil, approximately 55% of radioactivity was recovered in feces and approximately 42% in urine, with 15% of the dose excreted in urine as azilsartan. The elimination half-life of azilsartan is approximately 11 hours and renal clearance is approximately 2.3 mL/min. Steady-state levels of azilsartan are achieved within five days, and no accumulation in plasma occurs with repeated once-daily dosing. Azilsartan medoxomil is hydrolyzed to azilsartan, the active metabolite, in the gastrointestinal tract during absorption. Azilsartan medoxomil is not detected in plasma after oral administration. Dose proportionality in exposure was established for azilsartan in the azilsartan medoxomil dose range of 20 mg to 320 mg after single or multiple dosing. The estimated absolute bioavailability of azilsartan following administration of azilsartan medoxomil is approximately 60%. After oral administration of azilsartan medoxomil, peak plasma concentrations (Cmax) of azilsartan are reached within 1.5 to 3 hours. Food does not affect the bioavailability of azilsartan. For more Absorption, Distribution and Excretion (Complete) data for Azilsartan (8 total), please visit the HSDB record page. Metabolism / Metabolites Azilsartan medoxomil is rapidly hydrolysed to the active moiety azilsartan by esterases in the gastrointestinal tract and/or during drug absorption. Based on vitro studies, the enzymes involved in the hydrolysis of azilsartan medoxomil to azilsartan in human plasma, and in human liver and small intestinal cytosol seem to be similar to those involved in the hydrolysis of olmesartan medoxomil. Currently, no drug interactions are listed for the hydrolysis of azilsartan medoxomil. The enzyme carboxymethylenebutenolidase is a recently discovered hydrolysis mechanism for azilsartan medoxomi in the intestine and liver, but no interactions with other drugs have been reported for this enzyme in the Metabolism and Transport Drug Interaction Database (DIDB). Also no interactions have been reported for human serum albumin or arylesterases. Since there are multiple esterase pathways involved in the conversion of azilsartan medoxomil to azilsartan, the potential for interactions via this pathway is considered to be minimal. The metabolites M-I and M-II were formed by decarboxylation and dealkylation of azilsartan, respectively, and are pharmacologically inactive. CYP1A1, CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4 are all capable of metabolising azilsartan. However, CYP2C9 showed the highest activity in metabolising azilsartan to M-II and CYP2C8 in metabolising azilsartan to M-I. Azilsartan is metabolized to two primary metabolites. The major metabolite in plasma is formed by O-dealkylation, referred to as metabolite M-II, and the minor metabolite is formed by decarboxylation, referred to as metabolite M-I. Systemic exposures to the major and minor metabolites in humans were approximately 50% and less than 1% of azilsartan, respectively. M-I and M-II do not contribute to the pharmacologic activity of Edarbi. The major enzyme responsible for azilsartan metabolism is CYP2C9. Biological Half-Life The half-life of azilsartan in plasma was between 4 and 6 hr in rats and dogs and approximately 12 hr in humans. The elimination half-life of azilsartan is approximately 11 hours ... . |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Azilsartan is a white crystalline powder formulated into oral tablets. Azilsartan is an angiotensin II type 1 (AT1) receptor antagonist. It is used alone or in combination with other classes of antihypertensive agents in the management of hypertension. Azilsartan medoxomil, a prodrug, which is hydrolyzed to azilsartan in the gastrointestinal tract during absorption. HUMAN EXPOSURE AND TOXICITY: Limited data are available related to overdosage in humans. During controlled clinical trials in healthy subjects, once-daily doses up to 320 mg of azilsartan were administered for seven days and were well tolerated. The use of azilsartan during pregnancy is contraindicated. Drugs that act directly on the renin-angiotensin system (e.g., ACE inhibitors, angiotensin II receptor antagonists) reduce fetal renal function and increase fetal and neonatal morbidity and mortality when used in pregnancy during the second and third trimesters. ACE inhibitors also may increase the risk of major congenital malformations when administered during the first trimester of pregnancy. Azilsartan should be discontinued as soon as possible when pregnancy is detected, unless continued use is considered life-saving. ANIMAL STUDIES: There was no evidence of carcinogenicity when azilsartan was administered in the diet to mice and rats for up to two years. Also, azilsartan had no adverse effects on fertility of male or female rats at oral doses of up to 1,000 mg/kg/day. Azilsartan was not teratogenic when administered at oral doses up to 1,000 mg/kg/day to pregnant rats or up to 50 mg/kg/day to pregnant rabbits. However, embryo-fetal toxicity occurred at azilsartan doses of 1,000 mg/kg/day in rats (dilated renal pelvis and short supernumerary ribs) and 50 mg/kg/day in rabbits (increased post-implantation loss, embryo-fetal deaths, and decreased number of live fetuses). Embryo-fetal toxicity was also reported in rats with azilsartan doses as low as 30 mg/kg/day (delayed ossification in the caudal vertebrae) and 100 mg/kg/day (lower male fetal body weight) and at 500 mg/kg/day in rabbits (increased post-implantation loss). Azilsartan medoxomil, azilsartan, and the M-II metabolite were positive for structural aberrations in the Chinese Hamster Lung Cytogenetic Assay. In this assay, structural chromosomal aberrations were observed with the prodrug, azilsartan medoxomil, without metabolic activation. The active moiety, azilsartan was also positive in this assay both with and without metabolic activation. The major human metabolite, M-II was positive in this assay during a 24-hour assay without metabolic activation. Azilsartan medoxomil, azilsartan, and M-II were devoid of genotoxic potential in the Ames reverse mutation assay with Salmonella typhimurium and Escherichia coli, the in vitro Chinese Hamster Ovary Cell forward mutation assay, the in vitro mouse lymphoma (tk) gene mutation test, the ex vivo unscheduled DNA synthesis test, and the in vivo mouse and/or rat bone marrow micronucleus assay. Hepatotoxicity Azilsartan has been associated with a low rate of serum aminotransferase elevations that, in controlled trials, was no higher than with placebo therapy. These elevations were transient and rarely required dose modification. No specific instances of clinically apparent acute liver injury have been reported in association with azilsartan therapy, but it has been available for a limited time. Other ARBs have been linked to rare instances of symptomatic hepatotoxicity. The onset of liver injury is usually within 1 to 8 weeks of starting therapy and the serum enzyme pattern is typically hepatocellular with an acute hepatitis-like clinical syndrome. In some instances, cholestasis has developed which can be prolonged and relapsing, but ARB therapy has not been associated with vanishing bile duct syndrome or chronic liver injury. Immunoallergic manifestations (rash, fever, eosinophilia) are not common, nor is autoantibody formation. Likelihood score: E (Unproved but suspected rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Because no information is available on the use of azilsartan during breastfeeding, an alternate drug may be preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Interactions Since the use of potassium supplements and potassium-containing salt substitutes with an angiotensin II receptor antagonist (e.g., azilsartan medoxomil) can increase the potential for hyperkalemia, some clinicians have suggested that concomitant administration of these agents with azilsartan medoxomil should be avoided. Since the use of potassium-sparing diuretics (i.e., amiloride, spironolactone, triamterene) with an angiotensin II receptor antagonist (i.e., azilsartan medoxomil) can increase the potential for hyperkalemia, some clinicians have suggested that concomitant administration of these drugs with azilsartan medoxomil should be avoided. Concomitant treatment with nonsteroidal anti-inflammatory agents (NSAIAs), including selective cyclooxygenase-2 (COX-2) inhibitors, and angiotensin II receptor antagonists may result in deterioration of renal function, including possible acute renal failure, in patients who are geriatric, volume-depleted (including those on diuretic therapy), or have compromised renal function. These effects usually are reversible. Renal function should be periodically monitored in patients receiving azilsartan and NSAIA therapy. The antihypertensive effect of azilsartan may be attenuated in patients receiving NSAIAs, including selective COX-2 inhibitors. Reversible increases in serum creatinine, which may occur in patients receiving azilsartan medoxomil, may be larger in patients also receiving hydrochlorothiazide. 1. In vitro cytotoxicity: Azilsartan (TAK-536) exhibited cytotoxicity to HepG2 cells with an IC50 of 35 μM (48-hour treatment), but had no significant toxicity to normal human hepatocytes (L02 cells) at concentrations up to 80 μM [5] 2. Plasma protein binding: Azilsartan (TAK-536) had a plasma protein binding rate of >99% in human, rat, and dog plasma [1] 3. In vivo safety: In the 4-week Koletsky rat study and 8-week SHR study, Azilsartan (TAK-536) (up to 10 mg/kg/day) did not cause significant changes in liver function (ALT/AST) or kidney function (creatinine/BUN) [1][2] |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Edarbi is an angiotensin II receptor blocker (ARB) indicated for the treatment of hypertension to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes, including the class to which this drug principally belongs. /Included in US product label/ Edarbi may be used alone or in combination with other antihypertensive agents. Both angiotensin II receptor antagonists /eg, azilsartan/ and ACE inhibitors have been shown to slow the rate of progression of renal disease in hypertensive patients with diabetes mellitus and microalbuminuria or overt nephropathy, and use of a drug from either class is recommended in such patients. /NOT included in US product label/ Drug Warnings /BOXED WARNING/ WARNING: FETAL TOXICITY. When pregnancy is detected, discontinue Edarbi as soon as possibl. Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus Drugs that act directly on the renin-angiotensin system (e.g., ACE inhibitors, angiotensin II receptor antagonists) reduce fetal renal function and increase fetal and neonatal morbidity and mortality when used in pregnancy during the second and third trimesters. ACE inhibitors also may increase the risk of major congenital malformations when administered during the first trimester of pregnancy. Azilsartan should be discontinued as soon as possible when pregnancy is detected, unless continued use is considered life-saving. Nearly all women can be transferred successfully to alternative therapy for the remainder of their pregnancy. Use of drugs that affect the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue Edarbi as soon as possible. These adverse outcomes are usually associated with use of these drugs in the second and third trimester of pregnancy. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. Appropriate management of maternal hypertension during pregnancy is important to optimize outcomes for both mother and fetus. Because symptomatic hypotension may occur in patients with an activated renin-angiotensin system (e.g., patients with volume or salt depletion secondary to high doses of diuretics), azilsartan should be initiated in such patients after volume or salt depletion is corrected, or a lower initial dose of the drug should be used. If hypotension occurs in patients receiving azilsartan medoxomil, the patient should be placed in the supine position and, if necessary, an IV infusion of 0.9% sodium chloride injection should be administered. Transient hypotension is not a contraindication to additional doses of azilsartan, and therapy with the drug can be cautiously reinstated after blood pressure has been stabilized (e.g., with volume expansion). For more Drug Warnings (Complete) data for Azilsartan (14 total), please visit the HSDB record page. 1. Azilsartan (TAK-536) is a new-generation angiotensin II type 1 receptor blocker (ARB) with higher AT1R affinity and longer duration of action compared to traditional ARBs (e.g., losartan) [1][3] 2. Its pharmacological effects extend beyond blood pressure reduction: it improves insulin sensitivity (via upregulating GLUT4 and insulin signaling) [2], exerts neuroprotection (via SIRT3/PGC-1α-mediated mitochondrial protection) [4], and inhibits HepG2 cell proliferation (via ROS-NF-κB-apoptosis pathway) [5] 3. It is indicated for the treatment of essential hypertension, and preclinical studies suggest potential applications in insulin-resistant obesity and cerebral ischemic stroke [1][2][4] |
| 分子式 |
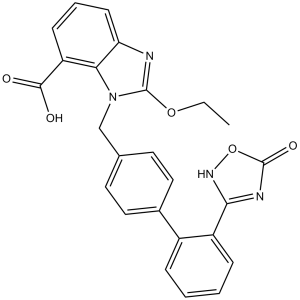
C25H20N4O5
|
|
|---|---|---|
| 分子量 |
456.45
|
|
| 精确质量 |
456.143
|
|
| CAS号 |
147403-03-0
|
|
| 相关CAS号 |
Azilsartan medoxomil;863031-21-4;Azilsartan-d5;1346599-45-8;Azilsartan-d4;1794817-45-0;Azilsartan medoxomil monopotassium;863031-24-7
|
|
| PubChem CID |
135415867
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.4±0.1 g/cm3
|
|
| 熔点 |
212-214 °C
|
|
| 折射率 |
1.695
|
|
| LogP |
4.21
|
|
| tPSA |
123.24
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
7
|
|
| 可旋转键数目(RBC) |
7
|
|
| 重原子数目 |
34
|
|
| 分子复杂度/Complexity |
783
|
|
| 定义原子立体中心数目 |
0
|
|
| InChi Key |
KGSXMPPBFPAXLY-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C25H20N4O5/c1-2-33-24-26-20-9-5-8-19(23(30)31)21(20)29(24)14-15-10-12-16(13-11-15)17-6-3-4-7-18(17)22-27-25(32)34-28-22/h3-13H,2,14H2,1H3,(H,30,31)(H,27,28,32)
|
|
| 化学名 |
2-ethoxy-3-[[4-[2-(5-oxo-4H-1,2,4-oxadiazol-3-yl)phenyl]phenyl]methyl]benzimidazole-4-carboxylic acid
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.48 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.48 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液添加到 900 μL 玉米油中并混合均匀。 View More
配方 3 中的溶解度: 30% PEG400+0.5% Tween80+5% Propylene glycol :30mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1908 mL | 10.9541 mL | 21.9082 mL | |
| 5 mM | 0.4382 mL | 2.1908 mL | 4.3816 mL | |
| 10 mM | 0.2191 mL | 1.0954 mL | 2.1908 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
None
CTID: jRCT1080222416
Phase: Status:
Date: 2014-03-05