| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 500μg |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
| 靶点 |
Influenza virus[1] Cap-dependent endonuclease (CEN)[1][2]
Influenza virus RNA polymerase PA subunit endonuclease: - For influenza A virus (H1N1, H3N2) PA endonuclease: The half-maximal inhibitory concentration (IC₅₀) was 0.3-0.8 μM [2] - For influenza B virus PA endonuclease: The IC₅₀ was 0.9-1.2 μM [2] - Recombinant influenza A virus (H5N1) PA endonuclease: The dissociation constant (Ki) was 0.15 μM [5] |
|---|---|
| 体外研究 (In Vitro) |
体外活性:Baloxavir(商品名 Xofluza;Baloxavir 酸或 BXA)是一种口服小分子帽依赖性核酸内切酶抑制剂,由 Roche 和 Shionogi 开发。它有效且选择性地抑制甲型和乙型流感病毒聚合酶 PA 亚基内的帽子依赖性核酸内切酶。 2018年2月,巴洛沙韦在日本获得全球首个批准,用于治疗甲型或乙型流感病毒感染。美国、欧盟和其他国家正在进行该适应症的 III 期开发。该药物通过抑制 mRNA 合成的起始来阻止流感病毒的增殖。 PA I38T 取代是降低 BXA 敏感性的主要途径,A 型病毒和 B 型病毒的 EC50 变化分别为 30 至 50 倍和 7 倍。带有 I38T 取代的病毒在细胞中表现出严重受损的复制适应性,并相应地降低了体外核酸内切酶活性。激酶测定:奥司他韦酸在 MES 测定缓冲液中连续稀释 [32.5 mmol/L MES 和 4 mmol/L CaCl2,溶于 DW(用 4 N NaOH 调节 pH 6.5)]。为了制备 NA 酶溶液,用 0.1% NP-40 灭活病毒原液,并用 MES 测定缓冲液稀释。 10 μL 奥司他韦酸溶液和 10 μL NA 酶溶液混合,37℃孵育 30 分钟,然后加入 30 μL 100 μmol/L 2'-(4-甲基伞形基)-α-DN -乙酰神经氨酸钠盐水合物(MUNANA;Sigma-Aldrich Co., Ltd.)。反应混合物在37℃下孵育60分钟,加入150μL终止液[0.1mol/L甘氨酸和25%乙醇(用4N NaOH调节pH 10.7)]终止反应。使用酶标仪EnVision 2103 (PerkinElmer Inc.)在激发波长355 nm和发射波长460 nm下测量荧光强度,然后使用XLfit软件计算IC50值。 FC是通过将每种测试病毒的IC50除以同源野生型病毒的IC50来计算的。细胞测定:犬肾MDCK细胞获自欧洲细胞培养物保藏中心。人准二倍体肿瘤RPMI2650和人胚胎肾293 T细胞由美国典型培养物保藏中心提供。 MDCK 和 RPMI2650 细胞维持在补充有 10% 胎牛血清 (FBS) 和 100 µg/mL 卡那霉素 (Thermo Fisher Scientific, Inc.) 的基本必需培养基 (MEM) 中。 293 T 细胞在含有 10% FBS 和 100 µg/mL 卡那霉素的 Dulbecco 改良 Eagle 培养基中培养。采用基于八个质粒的反向遗传学技术来产生所描述的重组病毒。 rgA/WSN/33 (H1N1) 质粒组和空载体 pHW2000 由 St. Jude 儿童研究医院的 Robert Webster 博士提供。用于生成 rgA/Victoria/3/75 和 rgB/Maryland 病毒的质粒是通过标准分子生物学技术用 pHW2000 构建的。所使用的引物序列可根据要求提供。 MDCK 和 293 T 细胞的共培养物用八种质粒转染并孵育 48 至 72 小时,然后在 MDCK 细胞中繁殖病毒。重组病毒的PA序列通过Sanger测序进行验证。通过MDCK细胞中的标准组织培养感染剂量(TCID)50测定或空斑形成单位(PFU)测定来测定病毒滴度。
抗甲型和乙型流感病毒活性: - 在感染甲型流感病毒(H1N1 pdm09株)的MDCK细胞中:Baloxavir(0.001-0.1 μM)呈剂量依赖性降低病毒产量,EC₅₀为0.008 μM;0.1 μM时,病毒产量较溶剂对照组降低约99% [2] - 在感染甲型流感病毒(H3N2株)的MDCK细胞中:EC₅₀为0.012 μM;0.1 μM Baloxavir抑制病毒复制约98% [2] - 在感染乙型流感病毒(山形系)的MDCK细胞中:EC₅₀为0.015 μM;0.1 μM Baloxavir降低病毒产量约97% [2] - 对奥司他韦耐药流感毒株的活性:在感染奥司他韦耐药甲型流感(H1N1)毒株(H275Y突变)的MDCK细胞中,Baloxavir仍表现出强效抗病毒活性,EC₅₀为0.009 μM,与对野生型毒株的活性相当 [6] - 抑制病毒mRNA合成:在感染甲型流感(H1N1)病毒的A549细胞中,Baloxavir(0.1 μM)在感染后8小时显著降低病毒M1和NP mRNA水平(qPCR检测),分别降低约85%和80%,证实其抑制病毒转录 [6] - 低细胞毒性:在MDCK、A549和HepG2细胞中,Baloxavir的半数细胞毒性浓度(CC₅₀)>10 μM,对所有测试流感毒株的选择指数(SI = CC₅₀/EC₅₀)>1000 [2] |
| 体内研究 (In Vivo) |
病毒神经氨酸酶抑制剂对感染人分离的H7N9甲型流感病毒的小鼠疗效有限。尽管巴洛沙韦可保护小鼠免受从人身上分离的低致病性H7N9禽流感病毒的致命攻击感染,但其对最近感染高致病性H7N9人分离株的小鼠的功效尚不清楚。本实验研究了巴洛沙韦对感染高致病性人H7N9病毒a /Guangdong/17SF003/2016的小鼠的疗效。用单次1.5 mg/kg剂量的巴洛沙韦治疗感染小鼠,保护小鼠免受高致病性人H7N9病毒感染的效果与奥司他韦50 mg/kg剂量的治疗相同,每天两次,连续5天。以15或50 mg/kg的剂量每日治疗5天,显示出卓越的治疗效果,在很大程度上阻止了病毒在呼吸器官中的复制。这些结果表明,巴洛沙韦是人类高致病性H7N9病毒感染患者有价值的候选治疗药物。[6]
在临床试验中,单剂量的巴洛沙韦可显着降低病毒滴度并减轻流感症状。 甲型流感(H1N1 pdm09株)感染小鼠模型的疗效: - 6-8周龄雄性ICR小鼠经鼻感染甲型流感病毒(100× LD₅₀)。Baloxavir以0.1、0.3、1 mg/kg剂量口服给药,每日1次,连续3天,从感染后1天开始。1 mg/kg剂量时,Baloxavir显著阻止体重下降(感染后7天体重变化:-5% vs 溶剂对照组-25%),并将存活率提高至100%(溶剂对照组存活率20%)[1][3] - 小鼠肺部病毒载量:感染后4天,1 mg/kg Baloxavir使肺部病毒滴度(空斑实验检测)从溶剂对照组的10⁶.⁵ PFU/g降至10².³ PFU/g [1][3] - 甲型流感(H1N1 pdm09株)感染雪貂模型的疗效: - 6-8月龄雌性雪貂经鼻感染甲型流感病毒。Baloxavir在感染后1天单次口服给药1 mg/kg。感染后3天,鼻洗液病毒滴度较溶剂对照组降低约10⁴倍;Baloxavir处理组雪貂无发热(体温<39.5°C),鼻分泌物减少 [6] - 降低雪貂体内流感病毒传播:在雪貂间传播模型中,对感染雪貂给予Baloxavir(1 mg/kg,口服,感染后1天)可减少病毒排出,使病毒向未感染接触雪貂的传播率降低50%(溶剂对照组传播率100%)[6] |
| 酶活实验 |
奥司他韦酸在 MES 测定缓冲液中连续稀释 [32.5 mmol/L MES 和 4 mmol/L CaCl2,溶于 DW(用 4 N NaOH 调节 pH 6.5)]。为了制备 NA 酶溶液,用 0.1% NP-40 灭活病毒原液,并用 MES 测定缓冲液稀释。 10 μL 奥司他韦酸溶液和 10 μL NA 酶溶液混合,37℃孵育 30 分钟,然后加入 30 μL 100 μmol/L 2'-(4-甲基伞形基)-α-DN -乙酰神经氨酸钠盐水合物(MUNANA;Sigma-Aldrich Co., Ltd.)。反应混合物在37℃下孵育60分钟,加入150μL终止液[0.1mol/L甘氨酸和25%乙醇(用4N NaOH调节pH 10.7)]终止反应。使用酶标仪EnVision 2103 (PerkinElmer Inc.)在激发波长355 nm和发射波长460 nm下测量荧光强度,然后使用XLfit软件计算IC50值。 FC是通过将每种测试病毒的IC50除以同源野生型病毒的IC50来计算的。
重组流感PA核酸内切酶活性测定(荧光底物法): - 反应体系为50 μL,含20 mM Tris-HCl(pH 7.5)、5 mM MgCl₂、1 mM DTT、0.1 mg/mL BSA、50 nM重组PA核酸内切酶及1 μM荧光标记DNA底物(模拟宿主mRNA的5'-帽结构)。Baloxavir浓度为0.01-10 μM。混合物37°C孵育60分钟后,检测荧光强度(激发485 nm,发射520 nm)以评估底物切割情况。通过绘制Baloxavir浓度与酶活性百分比(相对于溶剂对照组)的剂量-效应曲线,计算IC₅₀ [2] - PA核酸内切酶Ki值测定: - 采用上述相同反应体系,调整荧光底物浓度(0.25-2 μM)和Baloxavir浓度(0.05-0.5 μM),测定初始反应速率并绘制Lineweaver-Burk双倒数图。根据图中直线交点计算Ki值,证实Baloxavir对PA核酸内切酶的竞争性抑制作用 [5] |
| 细胞实验 |
犬肾 MDCK 细胞获自欧洲细胞培养物保藏中心。人准二倍体肿瘤RPMI2650和人胚胎肾293 T细胞由美国典型培养物保藏中心提供。 MDCK 和 RPMI2650 细胞维持在补充有 10% 胎牛血清 (FBS) 和 100 µg/mL 卡那霉素 (Thermo Fisher Scientific, Inc.) 的基本必需培养基 (MEM) 中。 293 T 细胞在含有 10% FBS 和 100 µg/mL 卡那霉素的 Dulbecco 改良 Eagle 培养基中培养。采用基于八个质粒的反向遗传学技术来产生所描述的重组病毒。 rgA/WSN/33 (H1N1) 质粒组和空载体 pHW2000 由 St. Jude 儿童研究医院的 Robert Webster 博士提供。用于生成 rgA/Victoria/3/75 和 rgB/Maryland 病毒的质粒是通过标准分子生物学技术用 pHW2000 构建的。所使用的引物序列可根据要求提供。 MDCK 和 293 T 细胞的共培养物用八种质粒转染并孵育 48 至 72 小时,然后在 MDCK 细胞中繁殖病毒。重组病毒的PA序列通过Sanger测序进行验证。通过MDCK细胞中的标准组织培养感染剂量(TCID)50测定或空斑形成单位(PFU)测定来测定病毒滴度。
MDCK细胞流感病毒感染及病毒产量测定: - MDCK细胞以5×10⁴个/孔接种于24孔板,过夜培养。细胞感染流感病毒(感染复数MOI=0.01),37°C孵育1小时。移除病毒接种液后,加入含Baloxavir(0.0001-10 μM)的培养基,37°C(5% CO₂)孵育48小时。收集上清液,通过MDCK细胞空斑实验测定病毒滴度。EC₅₀定义为使病毒滴度较溶剂对照组降低50%的Baloxavir浓度 [2] - A549细胞病毒mRNA qPCR检测: - A549细胞以2×10⁵个/孔接种于6孔板,感染甲型流感(H1N1)病毒(MOI=1),孵育1小时。细胞用Baloxavir(0.1 μM)或溶剂处理后,37°C继续孵育。感染后4、8、12小时提取总RNA,逆转录合成cDNA。采用病毒M1和NP基因特异性引物进行qPCR,以GAPDH为内参基因,通过2⁻ΔΔCt法计算相对病毒mRNA水平 [6] - 细胞毒性实验(MTT法): - MDCK、A549或HepG2细胞以1×10⁴个/孔接种于96孔板,过夜培养。细胞用Baloxavir(0.1-100 μM)或溶剂处理72小时。每孔加入10 μL MTT试剂(5 mg/mL),37°C孵育4小时。DMSO溶解甲臜结晶后,检测570 nm处吸光度。CC₅₀定义为使细胞活力降低50%的Baloxavir浓度 [2] |
| 动物实验 |
To evaluate the efficacy of baloxavir marboxil in vivo infection with the H7N9 virus, baloxavir marboxil was orally administered to mice at 5 and 50 mg/kg twice a day for five days and was shown to have completely protected them from lethal challenge infection with a low pathogenic avian H7N9 human isolate, A/Anhui/1/2013. Highly pathogenic A/Guangdong/17SF003/2016 virus, which possesses enhanced polymerase activity in mammals due to PB2-482R, PB2-588V, and PA-497R is more pathogenic than A/Anhui/1/2013 because it causes a systemic infection in mice, ferrets, and macaques; this greater pathogenicity may affect the efficacy of baloxavir marboxil. Although A/Guangdong/17SF003/2016 showed reduced growth in human bronchial epithelial cells, this virus possesses the A100V, R262K, V387I, N394D, I465V, and K497R mutations in PA that may affect sensitivity to baloxavir marboxil compared with A/Anhui/1/2013. Accordingly, here, we assessed the efficacy of baloxavir marboxil against this highly pathogenic human H7N9 virus in vitro and in vivo.[6]
Next, we assessed the efficacy of baloxavir marboxil in mice infected with the highly pathogenic human H7N9 virus. Six-week-old female mice (BALB/c, Japan SLC Inc.) were anesthetized with isoflurane and intranasally infected with 10 mouse lethal dose 50 (MLD50; 104.3 PFU) of highly pathogenic A/Guangdong/17SF003/2016 (H7N9) possessing NA-294R (arginine at position 294 of NA indicates sensitive to NA inhibitors). Five infected mice per group were orally treated with oseltamivir phosphate at 5 or 50 mg/kg twice a day for 5 days or with baloxavir marboxil at 1.5, 15, or 50 mg/kg once or twice a day for 5 days. The negative control mice received 0.5% methylcellulose because this reagent was used as a solvent. Body weight changes of these mice were monitored for 14 days and mice that lost 25% or more of their initial body weight were scored as dead and euthanized according to institutional guidelines. All animal experiments were conducted in accordance with the University of Tokyo’s Regulations for Animal Care and Use, which were approved by the Animal Experiment Committee of the Institute of Medical Science, the University of Tokyo (approval no. PA15-12). Mice given methylcellulose exhibited immediate body weight loss and died up to 8 days after infection. Oseltamivir phosphate treatment at 5 mg/kg for 5 days slightly improved the survival time of the infected mice (p = 0.009, log-rank test) but failed to protect them from the lethal challenge infection. Oseltamivir phosphate treatment at 50 mg/kg for 5 days showed 80% protection with severe body weight loss. In contrast, 60% of mice treated once with baloxavir marboxil at 1.5 mg/kg survived for 14 days, whereas all of the mice in the other baloxavir-treated groups survived without any body weight loss (p = 0.0016, log-rank test). These results show that a single dose of baloxavir marboxil with 15 mg/kg is sufficient to protect mice from infection with a highly pathogenic human H7N9 virus[6]. Mouse influenza infection model: - Male ICR mice (20-25 g, 6-8 weeks old) were randomly divided into 4 groups (n=10 per group): vehicle control and Baloxavir (0.1, 0.3, 1 mg/kg). Baloxavir was dissolved in a solution of 0.5% methylcellulose and 0.1% Tween 80 in distilled water. Mice were anesthetized with isoflurane and intranasally infected with influenza A (H1N1 pdm09) virus (100 μL, 100× LD₅₀). Baloxavir was administered orally via gavage once daily for 3 days, starting at 24 hours post-infection (vehicle group received the same volume of solvent). Body weight and survival were monitored daily for 14 days. On day 4 post-infection, 3 mice per group were euthanized, and lungs were excised to measure virus titer via plaque assay [1][3] - Ferret influenza infection and transmission model: - Female ferrets (1-1.5 kg, 6-8 months old) were acclimated for 1 week before experiments. Ferrets were anesthetized with ketamine/xylazine and intranasally infected with influenza A (H1N1 pdm09) virus (1 mL, 10⁶ PFU). Baloxavir was dissolved in 0.5% methylcellulose/0.1% Tween 80 and administered orally at 1 mg/kg once on day 1 post-infection (vehicle group received solvent). Nasal washes were collected daily for 7 days to measure virus titer via plaque assay. Body temperature and clinical signs (nasal discharge, sneezing) were recorded. For transmission experiments, infected ferrets (treated with Baloxavir or vehicle) were housed with naive ferrets (1:1 ratio) for 7 days; nasal washes of naive ferrets were tested for virus to determine transmission rate [6] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Following oral administration of 40 mg baloxavir marboxil in adolescents and adults aged 12 years and older, the AUC was 5520 ng x hr/mL and the Cmax was 68.9 ng/mL. Following a 80 mg dose, the the AUC was 6930 ng x hr/mL and the Cmax was 82.5 ng/mL. The Tmax is about four hours. Food decreased Cmax by 48% and AUC0-inf by 36%. In pediatric patients aged five to 12 years of age weighing less than 20 kg, the AUCinf was 5830 ng x hr/mL and the Cmax was 148 ng/mL following a 2 mg/kg dose. The AUCinf was 4360 ng x hr/mL and the Cmax was 81.1 ng/mL following a 40 mg dose in pediatric patients who weigh greater than or equal to 20 kg. The Tmax ranged from 3.5 to 4.5 hours. Baloxavir is primarily eliminated by biliary excretion. About 80.1% of the total dose is excreted in feces. About 14.7% of the dose is excreted in urine, where 3.3% of the recovered dose is the unchanged parent drug. The volume of distribution is 1180 L. Clearance of baloxavir is 10.3 L/h. Metabolism / Metabolites Baloxavir predominantly undergoes UGT1A3-mediated metabolism to form glucuronic acid conjugate. It is subsequently metabolized by CYP3A4 to form sulfoxide. Biological Half-Life The apparent terminal elimination half-life of baloxavir is 79.1 hours. Oral bioavailability: In mice, oral administration of Baloxavir (1 mg/kg) resulted in an oral bioavailability of ~70% [5] - Plasma pharmacokinetics in mice: After oral administration of Baloxavir (1 mg/kg), the maximum plasma concentration (Cmax) was 1.2 μM, and the area under the plasma concentration-time curve (AUC₀₋₂₄h) was 8.5 μM·h; the elimination half-life (t₁/₂) was ~6 hours [5] - Tissue distribution: In mice treated with oral Baloxavir (1 mg/kg), drug concentrations in the lungs and nasal mucosa (target sites of influenza infection) were 2.5 μM and 3.1 μM at 2 hours post-administration, respectively, which were higher than the plasma concentration (1.2 μM) [1][3] - Metabolism: In human liver microsomes, Baloxavir was minimally metabolized; less than 10% of the parent drug was converted to metabolites after 2 hours of incubation, indicating low reliance on hepatic cytochrome P450 enzymes [4] |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In clinical trials, there was little evidence that baloxavir caused liver injury, either in the form of serum enzyme elevations or clinically apparent liver disease. A proportion of patients with acute influenza A may have minor serum enzyme elevations during the acute illness, but these are independent of therapy and do not appear to be exacerbated by baloxavir. Likelihood score: E (unlikely cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the use of baloxavir marboxil during breastfeeding. Because baloxavir is 93% bound to plasma proteins, the amount in milk is likely to be low. If baloxavir is required by the mother, it is not a reason to discontinue breastfeeding, but an alternate drug may be preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Acute toxicity: In mice, oral administration of Baloxavir at doses up to 200 mg/kg did not cause mortality or significant clinical signs (e.g., lethargy, weight loss) within 14 days [5] - Subacute toxicity: In rats treated with oral Baloxavir (10, 30, 100 mg/kg/day) for 28 days, no significant changes were observed in body weight, food consumption, or serum biochemical parameters (ALT, AST, BUN, creatinine) compared to the control group [5] - Plasma protein binding: In human plasma, the plasma protein binding rate of Baloxavir was ~90% [4] - Drug-drug interactions: Co-administration of Baloxavir with oseltamivir (another anti-influenza drug) in mice did not affect the plasma concentrations of either drug, and no synergistic or antagonistic toxicity was observed [6] |
| 参考文献 | |
| 其他信息 |
Baloxavir is under investigation in clinical trial NCT04327791 (Combination Therapy With Baloxavir and Oseltamavir 1 for Hospitalized Patients With Influenza (The COMBO Trial 1)).
Baloxavir is a Polymerase Acidic Endonuclease Inhibitor. The mechanism of action of baloxavir is as a Polymerase Acidic Endonuclease Inhibitor, and Chelating Activity. Baloxavir is an inhibitor of the influenza cap-dependent endonuclease enzyme and is used as therapy of influenza A and B. Baloxavir is given as a single, one-time dose and has not been associated with serum enzyme elevations or with clinically apparent liver injury. See also: Baloxavir Marboxil (active moiety of). Baloxavir acid (BXA), derived from the prodrug baloxavir marboxil (BXM), potently and selectively inhibits the cap-dependent endonuclease within the polymerase PA subunit of influenza A and B viruses. In clinical trials, single doses of BXM profoundly decrease viral titers as well as alleviating influenza symptoms. Here, we characterize the impact on BXA susceptibility and replicative capacity of variant viruses detected in the post-treatment monitoring of the clinical studies. We find that the PA I38T substitution is a major pathway for reduced susceptibility to BXA, with 30- to 50-fold and 7-fold EC50 changes in A and B viruses, respectively. The viruses harboring the I38T substitution show severely impaired replicative fitness in cells, and correspondingly reduced endonuclease activity in vitro. Co-crystal structures of wild-type and I38T influenza A and B endonucleases bound to BXA show that the mutation reduces van der Waals contacts with the inhibitor. A reduced affinity to the I38T mutant is supported by the lower stability of the BXA-bound endonuclease. These mechanistic insights provide markers for future surveillance of treated populations.[1] Cap-dependent endonuclease (CEN) resides in the PA subunit of the influenza virus and mediates the critical "cap-snatching" step of viral RNA transcription, which is considered to be a promising anti-influenza target. Here, we describe in vitro characterization of a novel CEN inhibitor, baloxavir acid (BXA), the active form of baloxavir marboxil (BXM). BXA inhibits viral RNA transcription via selective inhibition of CEN activity in enzymatic assays, and inhibits viral replication in infected cells without cytotoxicity in cytopathic effect assays. The antiviral activity of BXA is also confirmed in yield reduction assays with seasonal type A and B viruses, including neuraminidase inhibitor-resistant strains. Furthermore, BXA shows broad potency against various subtypes of influenza A viruses (H1N2, H5N1, H5N2, H5N6, H7N9 and H9N2). Additionally, serial passages of the viruses in the presence of BXA result in isolation of PA/I38T variants with reduced BXA susceptibility. Phenotypic and genotypic analyses with reverse genetics demonstrate the mechanism of BXA action via CEN inhibition in infected cells. These results reveal the in vitro characteristics of BXA and support clinical use of BXM to treat influenza. [2] Baloxavir marboxil (Xofluza™; baloxavir) is an oral cap-dependent endonuclease inhibitor that has been developed by Roche and Shionogi. The drug blocks influenza virus proliferation by inhibiting the initiation of mRNA synthesis. In February 2018, baloxavir received its first global approval in Japan for the treatment of influenza A or B virus infections. Phase III development is underway in the USA, EU and other countries for this indication. This article summarized the milestones in the development of baloxavir leading to this first global approval for influenza A or B virus infections.[4] Baloxavir (Xofluza; BXA; S033447) is a novel cap-dependent endonuclease inhibitor targeting the PA subunit of influenza virus RNA polymerase, developed for the treatment of influenza A and B virus infections [4][5] - Mechanism of action: Baloxavir inhibits the PA endonuclease activity of influenza virus, which is essential for the synthesis of viral mRNA (the enzyme cleaves the 5'-cap structure of host mRNA to prime viral mRNA synthesis). By blocking this step, Baloxavir suppresses viral transcription and replication [2][6] - Clinical relevance: Baloxavir has a long half-life and high tissue penetration in respiratory tissues, allowing for single-dose oral administration. It is effective against oseltamivir-resistant influenza strains, addressing the challenge of influenza drug resistance [4][6] - Regulatory status: Baloxavir was approved for the treatment of uncomplicated influenza A and B virus infections in adults and pediatric patients (≥12 years old) in multiple countries, including the United States and Japan, by 2018 [4] |
| 分子式 |
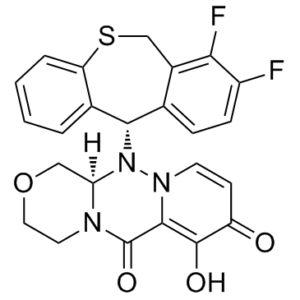
C₂₄H₁₉F₂N₃O₄S
|
|---|---|
| 分子量 |
483.49
|
| 精确质量 |
483.106
|
| 元素分析 |
C, 59.62; H, 3.96; F, 7.86; N, 8.69; O, 13.24; S, 6.63
|
| CAS号 |
1985605-59-1
|
| 相关CAS号 |
Baloxavir marboxil;1985606-14-1;Baloxavir-d5;Baloxavir-d4;2415027-80-2
|
| PubChem CID |
124081876
|
| 外观&性状 |
White to yellow solid powder
|
| 密度 |
1.6±0.1 g/cm3
|
| 沸点 |
644.7±65.0 °C at 760 mmHg
|
| 闪点 |
343.7±34.3 °C
|
| 蒸汽压 |
0.0±2.0 mmHg at 25°C
|
| 折射率 |
1.757
|
| LogP |
1.51
|
| tPSA |
98.6Ų
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
9
|
| 可旋转键数目(RBC) |
1
|
| 重原子数目 |
34
|
| 分子复杂度/Complexity |
932
|
| 定义原子立体中心数目 |
2
|
| SMILES |
S1CC2C(=C(C=CC=2[C@@H](C2C=CC=CC1=2)N1[C@@H]2COCCN2C(C2=C(C(C=CN12)=O)O)=O)F)F
|
| InChi Key |
FIDLLEYNNRGVFR-CTNGQTDRSA-N
|
| InChi Code |
InChI=1S/C24H19F2N3O4S/c25-16-6-5-13-15(20(16)26)12-34-18-4-2-1-3-14(18)21(13)29-19-11-33-10-9-27(19)24(32)22-23(31)17(30)7-8-28(22)29/h1-8,19,21,31H,9-12H2/t19-,21+/m1/s1
|
| 化学名 |
({(12aR)-12-[(11S)-7,8-difluoro-6,11-dihydrodibenzo[b,e]thiepin-11-yl]-6,8-dioxo-3,4,6,8,12,12ahexahydro-1H-[1,4]oxazino[3,4-c]pyrido[2,1-f][1,2,4]triazin-7-yl}oxy)
|
| 别名 |
Trade name Xofluza; Baloxavir acid; BXA; Baloxavir acid; 4G86Y4JT3F; S-033447; UNII-4G86Y4JT3F; (3R)-2-[(11S)-7,8-difluoro-6,11-dihydrobenzo[c][1]benzothiepin-11-yl]-11-hydroxy-5-oxa-1,2,8-triazatricyclo[8.4.0.03,8]tetradeca-10,13-diene-9,12-dione; Baloxavir (USAN); Baloxavir marboxil; S-033447; S 033447; S033447; Baloxavir;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : 25~41.67 mg/mL ( 51.7~86.19 mM )
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (4.30 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (4.30 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 View More
配方 3 中的溶解度: 10% DMSO+40% PEG300+5% Tween-80+45% Saline : ≥ 2.08 mg/mL (4.30 mM) 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0683 mL | 10.3415 mL | 20.6830 mL | |
| 5 mM | 0.4137 mL | 2.0683 mL | 4.1366 mL | |
| 10 mM | 0.2068 mL | 1.0341 mL | 2.0683 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05012189 | Active, not recruiting | Drug: Oseltamivir Drug: Baloxavir |
Influenza Influenza, Human | Insight Therapeutics, LLC | August 6, 2021 | Phase 4 |
| NCT06094010 | Recruiting | Drug: Baloxavir Marboxil | Influenza | Hoffmann-La Roche | November 22, 2023 | Phase 3 |
| NCT03969212 | Recruiting | Drug: Baloxavir Marboxil Drug: Placebo |
Influenza | Hoffmann-La Roche | October 10, 2019 | Phase 3 |
| NCT04327791 | Recruiting | Drug: Baloxavir Drug: Placebos |
Influenza | Bassett Healthcare | April 3, 2020 | Phase 2 Phase 3 |
 In vitroendonuclease activity and inhibition of PA variants and thermal stabilization induced by the binding of BXA.Sci Rep.2018 Jun 25;8(1):9633. |
|---|
 BXA binding to influenza A/H1N1 PA endonuclease. BXA interacts with (A) PA-A WT and (B) PA-A I38T by chelating the two manganese ions in the active site.Sci Rep.2018 Jun 25;8(1):9633. |
 Comparison of PA endonuclease from Flu A and Flu B bound to BXA in either WT or I38T form. Superposition of PA-BXA complexes: (A) PA-A WT and PA-A I38T, (B) PA-B WT and PA-B I38T, (C) PA-A WT and PA-B WT, (D) PA-A I38T and PA-B I38T.Sci Rep.2018 Jun 25;8(1):9633. |
 Local interactions of residue 38 in apo- and BXA-bound FluB PA (A) Superposition of ligand-free PA-B WT (PDB:5FML, in hotpink) and bound to BXA (green sticks for BXA, teal sticks/cartoon for PA). (B) Superposition of ligand-free (forest green) and BXA-bound PA-B I38T (light magenta sticks for BXA, orange sticks/cartoon for PA).Sci Rep.2018 Jun 25;8(1):9633. |
 Replicative capacity of variant viruses with indicated AA substitutions in PA protein. Canine MDCK cells (A–C) or human RPMI2650 cells (D,E) were infected with WT or I38x viruses based on rgA/WSN/33 (H1N1) (A,D), rgA/Victoria/3/75 (H3N2) (B,E), or B/Maryland/1/5 |