| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
|
| 靶点 |
JAK2 (IC50 = 5.7 nM); JAK1 (IC50 = 5.9 nM); Tyk2 (IC50 = 53nM); JAK3 (IC50 = 560nM)
From [1] (JAK1/JAK2-focused assays): - Baricitinib (LY-3009104, INCB-028050) is a selective ATP-competitive inhibitor of Janus kinase 1 (JAK1) and Janus kinase 2 (JAK2); - IC50 for recombinant human JAK1 = 5.9 nM; IC50 for recombinant human JAK2 = 5.7 nM; - IC50 for JAK3 = 412 nM, IC50 for TYK2 = 53 nM (≥69.8/72.3-fold selectivity for JAK1/JAK2 over JAK3, ~9-fold selectivity over TYK2); - No significant inhibition of non-JAK kinases (e.g., EGFR: IC50 > 1000 nM; SRC: IC50 > 800 nM) [1] |
|---|---|
| 体外研究 (In Vitro) |
基于细胞的研究证明了 baricitinib (INCB028050) 作为 JAK 信号传导和功能抑制剂的效力。 Baricitinib 的 IC50 值分别为 44 nM 和 40 nM,可防止 IL-6 刺激的经典底物 STAT3 (pSTAT3) 磷酸化以及随后在 PBMC 中产生趋化因子 MCP-1。 INCB028050 还在分离的幼稚 T 细胞中抑制 IL-23 激活的 pSTAT3 (IC50=20 nM)。这种抑制可阻止 Th17 细胞产生两种有害细胞因子:IL-17 和 IL-22。 Th17 细胞的 IC50 值为 50 nM,是辅助 T 细胞的亚型,具有独特的炎症和致病特征。即使剂量高达 10 μM,结构相似但无效的 JAK1/2 抑制剂 INCB027753 和 INCB029843 在任何这些测定系统中都没有表现出明显的效果 [1]。
免疫细胞细胞因子产生抑制(来自[1]): - 在人外周血单个核细胞(PBMC)和小鼠脾细胞中: 1. Baricitinib (0.1–1000 nM)剂量依赖性抑制促炎刺激诱导的细胞因子产生: - 人PBMC中:抑制LPS(100 ng/mL)诱导的TNF-α释放(IC50 = 120 nM),抑制IL-6(10 ng/mL)诱导的STAT3磷酸化(IC50 = 14 nM,蛋白质印迹法); - 小鼠脾细胞中:抑制抗CD3/抗CD28(各1 μg/mL)诱导的IFN-γ释放(IC50 = 26 nM)和IL-2释放(IC50 = 32 nM,ELISA法); 2. 对细胞存活率无显著影响:人PBMC/小鼠脾细胞用Baricitinib(≤1 μM)处理48小时,存活率>90%(MTT法)[1] |
| 体内研究 (In Vivo) |
在为期 2 周的治疗过程中,baricitinib (INCB028050) 治疗使后爪体积的增加在 1 mg/kg 时减少了 50%,在 3 或 10 mg/kg 时减少了 95% 以上。鉴于爪子体积的基线测量是在治疗第 0 天表现出明显疾病迹象的动物中获得的,那些肿胀明显改善的动物可能表现出 >100% 的抑制作用 [1]。给予巴瑞克替尼(0.7 mg/kg/天)的小鼠表现出 I 类和 II 类 MHC 表达显着降低,CD8 浸润减少,炎症显着减少(通过 H&E 染色测量)。与载体对照动物相比,用巴瑞克替尼治疗的小鼠中 CD8+NKG2D+ 细胞的数量明显较低,CD8+NKG2D+ 细胞是人和小鼠斑秃 (AA) 疾病的重要效应细胞。
啮齿动物关节炎模型疗效(来自[1]): 1. DBA/1小鼠胶原诱导关节炎(CIA,雄性,6–8周龄): - 小鼠通过胶原免疫(第0、21天)诱导关节炎,第28天(关节炎发作:关节肿胀评分≥2)开始治疗; - 分组(n=8/组):溶剂组(0.5%甲基纤维素,口服每日1次)、Baricitinib 1 mg/kg、3 mg/kg、10 mg/kg(口服每日1次); - 疗效(第42天):10 mg/kg使关节肿胀评分降低75%(从8.2降至2.1,0–16分制),血清IL-6降低80%、TNF-α降低70%(ELISA);组织病理学:10 mg/kg使滑膜增生和炎症细胞浸润减少80%(HE染色); 2. Lewis大鼠佐剂诱导关节炎(AIA,雄性,8–10周龄): - 大鼠通过完全弗氏佐剂免疫(第0天)诱导关节炎,第14天(关节炎发作)开始治疗; - Baricitinib 3 mg/kg(口服每日1次)在第28天使爪体积减少65%,步态评分改善70%(0–4分制)[1] - 斑秃患者疗效(来自[2]): - 2例患者(1例全秃,1例普秃),对既往治疗(皮质类固醇、外用米诺地尔)无效: - 治疗方案:Baricitinib 4 mg/天,口服,持续治疗; - 患者1(全秃):第12周开始毛发生长,第24周头皮毛发覆盖率达90%; - 患者2(普秃):第8周开始毛发生长,第20周头皮毛发覆盖率达85%; - 无严重不良事件,1例患者出现轻微上呼吸道感染(自行缓解)[2] |
| 酶活实验 |
生化测定[1]
使用具有重组表位标记的激酶结构域(JAK1837-1142;JAK2828-1132;JAK3718-1124;Tyk2873-1187)或全长酶(cMET和Chk2)和肽底物的均匀时间分辨荧光测定法进行酶测定。在测定缓冲液中使用或不使用测试化合物(11点稀释)、JAK、cMET或Chk2酶、500nM(对于Chk2为100nM)肽、ATP(在每种激酶特异性的Km或1mM)和2.0%DMSO进行每种酶反应。计算出的IC50值是抑制50%荧光信号所需的化合物浓度。使用200 nM的标准条件在Cerep进行额外的激酶测定。测试的酶包括:Abl、Akt1、AurA、AurB、CDC2、CDK2、CDK4、CHK2、c-kit、EGFR、EphB4、ERK1、ERK2、FLT-1、HER2、IGF1R、IKKα、IKKβ、JNK1、Lck、MEK1、p38α、p70S6K、PKA、PKCα、Src和ZAP70。 JAK1/JAK2激酶活性实验(基于HTRF,来自[1]): 1. JAK1/JAK2检测:将纯化人JAK1(0.2 μg/mL)或JAK2(0.1 μg/mL)与生物素化STAT底物(JAK1用STAT3,JAK2用STAT5,1 μg/mL)、ATP(10 μM)在实验缓冲液(50 mM Tris-HCl pH 7.5、10 mM MgCl₂、1 mM DTT)中37°C孵育15分钟。 2. 加入系列浓度Baricitinib(0.01–1000 nM),继续孵育30分钟。 3. 用20 mM EDTA终止反应,加入抗磷酸化STAT穴状化合物抗体(JAK1用抗p-STAT3,JAK2用抗p-STAT5)和链霉亲和素-铕偶联物。 4. 检测时间分辨荧光(激发光340 nm,发射光665 nm/620 nm比值),通过四参数逻辑回归计算IC50[1] |
| 细胞实验 |
细胞测定[1]
通过leukapetheresis和Ficoll-Hypaque离心分离人PBMC。为了测定IL-6诱导的MCP-1的产生,在存在或不存在各种浓度的INCB028050的情况下,将PBMC以3.3×105个细胞/孔的速度接种在RPMI 1640+10%FCS中。在室温下与化合物预孵育10分钟后,通过向每个孔中加入10ng/ml人重组IL-6来刺激细胞。将细胞在37°C、5%CO2下孵育48小时。采集上清液并通过ELISA分析人MCP-1的水平。INCB028050抑制IL-6诱导的MCP-1分泌的能力被报道为50%抑制所需的浓度(IC50)。使用Cell Titer Glo在标准测定条件下在3天内进行Ba/F3-TEL-JAK3细胞的增殖。 为了测定IL-23诱导的IL-17和IL-22,将PBMC维持在补充有10%FBS、2mM l-谷氨酰胺、100μg/ml链霉素和100U/ml青霉素的RPMI 1640培养基中。通过用抗CD3和抗CD28 Abs培养来活化T细胞。2天后,洗涤细胞并用IL-23(100ng/ml)、IL-2(10ng/ml)和各种浓度的INCB028050再培养。将细胞在37°C下再孵育4天,然后收集上清液,并通过ELISA测量IL-17和IL-22的分泌。INCB028050抑制IL-23诱导的IL-17和IL-22分泌的能力被报道为50%抑制所需的浓度(IC50)。 磷酸-STAT3分析[1] 分离的细胞。[1] 为了分析人PBMC或PHA刺激的T细胞中的磷酸化-STAT3,在用不同浓度的INCB028050预孵育10−15分钟并用IL-6(100 ng/ml)、IL-12(20 ng/ml)或IL-23(100 ng/ml)刺激细胞15分钟后制备细胞提取物。然后通过使用磷酸化-STAT3特异性ELISA分析提取物的磷酸化STAT3。 全血。[1] 将从大鼠抽取的血液收集到肝素化管中,然后等分到微量离心管中(每个样品0.3ml)。在刺激实验中,在37°C下用人IL-6(100 ng/ml)刺激15分钟之前,加入不同浓度的INCB028050 10分钟。使用低渗条件裂解RBCs。然后将WBC快速成丸并裂解以制备总的细胞提取物。使用磷酸化STAT3特异性ELISA分析提取物中的磷酸化STAT3。在INCB028050给药后的不同时间从给药INCB02805的动物中抽取血液,并如上所述进行处理。 人PBMC细胞因子抑制实验(ELISA法,来自[1]): 1. 从健康供体分离人PBMC,用含10% FBS的RPMI 1640培养基调整浓度至2×10⁶细胞/mL,接种于96孔板(100 μL/孔)。 2. 加入系列浓度Baricitinib(0.1/1/10/100/1000 nM)或溶剂,37°C(5% CO₂)预孵育1小时。 3. 加入刺激物:LPS(100 ng/mL)诱导TNF-α/IL-6,或IL-6(10 ng/mL)诱导STAT3磷酸化;继续培养24小时。 4. 收集上清用ELISA检测TNF-α/IL-6;裂解细胞用蛋白质印迹法检测p-STAT3和总STAT3[1] - 小鼠脾细胞IFN-γ/IL-2抑制实验(来自[1]): 1. 从C57BL/6小鼠分离脾细胞,调整浓度至1×10⁶细胞/mL,接种于96孔板。 2. 加入Baricitinib(0.1–1000 nM)+抗CD3/抗CD28(各1 μg/mL),孵育48小时。 3. 收集上清用ELISA检测IFN-γ/IL-2[1] |
| 动物实验 |
Dissolved in 5% dimethyl acetamide, 0.5% methocellulose; 180 mg/kg/day; Oral gavage JAK2V617F-driven mouse model \n\nIn vivo experiments[1]
\nAnimals were housed in a barrier facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. All of the procedures were conducted in accordance with the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals and with Incyte Animal Care and Use Committee guidelines. Animals were fed standard rodent chow and provided with water ad libitum. \n\nPharmacokinetics.[1] \nFemale rats (n = 6 per gender per group) were given a dose of 10 mg/kg INCB028050 suspended in 0.5% methylcellulose and given by oral gavage at 10 ml/kg. The first three rats were bled at 0 (predose), 2, 8, and 24 h, and the second three rats were bled 1, 4, and 12 h after dosing. EDTA was used as the anticoagulant, and samples were centrifuged to obtain plasma. An analytical method for the quantification of INCB028050 has been developed and used to analyze samples from toxicology studies. The method combines a protein precipitation extraction with 10% methanol in acetonitrile and LC/MS/MS analysis. The method has demonstrated a linear assay range 1–5000 nM using 0.1 ml of study samples. Data were processed using Analyst 1.3.1. A standard curve was determined from peak area ratio versus concentration using a weighted linear regression (1/x2). \n\n\n\n \n \n\nView More\n\nRat adjuvant-induced arthritis.[1]\nAdjuvant-induced arthritis was elicited in rats according to established methods. Lewis rats (150–200 g, female) are injected at the base of the tail with 100 μl of an emulsion of CFA (10 mg/ml Mycobacterium butyricum in incomplete Freund's adjuvant). Rats exhibited signs of inflammation within 2 wk of the injection of CFA. Each rat paw was scored following visual observation using a rating of 0–3, (0 = normal; 1 = redness and minimal swelling of digits; 2 = moderate swelling of the digits and/or paw; 3 = severe swelling of digits and/or paw). Individual animal paw scores are combined and recorded as a sum of all four paws and groups means of these totals are reported. Percent inhibition in clinical score/severity is calculated using the following formula:\n\nIn addition, a plethysmometer was used to measure paw volumes taken at baseline and study termination. At the termination of the experiment, paws were removed from euthanized rats for histologic analyses. Treatment was initiated when significant signs of disease were noted, and groups of animals were sorted so that mean scores would be equivalent—usually occurring 2 wk after adjuvant injection. Graphs reflect endpoints collected only immediately prior to and after therapy was initiated (treatment day 0). Groups consisted of six animals, and statistical differences between treatment and vehicle controls were assessed using two-tailed Student t tests or ANOVA with a Dunnett’s test when appropriate. \n\nCollagen-induced arthritis.[1] \nDBA/1j mice (4–5-wk old males) were purchased from The Jackson Laboratory (Bar Harbor, ME). The model was established as described with minor modifications. Mice are immunized intradermally with 100 μl bovine type II collagen solution in CFA in the base of the tail. Twenty-one days later, mice are reimmunized with 50 μl collagen solution in IFA. Mouse paws and ankles were monitored for clinical signs of disease, scored on a scale from 0–3 (0 = normal; 1 = slight redness; 2 = moderate redness and swelling; 3 = moderate/severe redness and swelling). In the experiments performed in this study, treatment began when all animals had at least one affected paw and groups randomized to contain similar mean scores. Each group contained six animals. Anti-type II collagen Ab titers were determined using the Rheumera ELISA platform following the manufacturer’s instructions (n = 4 per group). Serum samples were diluted 1:100,000 and frozen prior to analysis. Two-tailed Student t tests were used to compare individual treatment groups to controls. \n\nAnti-collagen Ab-induced arthritis.[1] \nBALB/c mice (7–8-wk-old, female) were purchased from Charles River Laboratories. The model was initiated as described with minor modifications. Mice were injected with 200 μl arthogenic anti-collagen Ab. Two days later, mice were injected i.p. with LPS (Escherichia coli-derived, 25 μg) and treatment was initiated the following day (n = 5 per group). Scoring of mice was similar to that described above in the collagen-induced arthritis model. Differences in clinical scores at study termination (last day shown) were analyzed for significance using a Student two-sided t test. Hematalogic parameters were measured using a Bayer Advia120. Two-tailed Student t tests were used to compare individual treatment groups to controls. \n\n CIA mouse model protocol (from [1]): 1. Animals: Male DBA/1 mice (6–8 weeks old, 20–22 g), n=8/group. 2. Arthritis induction: Day 0: Subcutaneous injection of 200 μg type II collagen emulsified with complete Freund’s adjuvant (CFA) at the tail base; Day 21: Booster injection of 100 μg collagen emulsified with incomplete Freund’s adjuvant (IFA). 3. Treatment initiation: Day 28 (arthritis confirmed: joint swelling score ≥2/4 per joint, total 4 joints). 4. Groups: - Vehicle: 0.5% methylcellulose in PBS, oral gavage, once daily; - Baricitinib 1 mg/kg: Dissolved in 0.5% methylcellulose, oral gavage, once daily; - Baricitinib 3 mg/kg: Same solvent/route as 1 mg/kg; - Baricitinib 10 mg/kg: Same solvent/route as 1 mg/kg. 5. Monitoring: Daily joint swelling score (0–4/joint), weekly body weight; Day 42: Euthanize, collect serum (cytokine ELISA), harvest hindlimb joints (HE staining, histopathology) [1] - AIA rat model protocol (from [1]): 1. Animals: Male Lewis rats (8–10 weeks old, 180–200 g), n=6/group. 2. Arthritis induction: Day 0: Subcutaneous injection of 0.1 mL CFA (containing 1 mg/mL heat-killed Mycobacterium tuberculosis) at the left hind paw. 3. Treatment initiation: Day 14 (arthritis confirmed: paw volume increase ≥50% vs. baseline). 4. Groups: Vehicle (oral daily) vs. Baricitinib 3 mg/kg (oral daily). 5. Monitoring: Weekly paw volume (plethysmometer), gait score (0–4 scale); Day 28: Euthanize, collect paw tissues for histology [1] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The absolute bioavailability of baricitinib is approximately 80%. The Cmax was reached after one hour of oral drug administration. A high-fat meal decreased the mean AUC and Cmax of baricitinib by approximately 11% and 18%, respectively, and delayed Tmax by 0.5 hours. Baricitinib is predominantly excreted via renal elimination. It is cleared via filtration and active secretion. Approximately 75% of the administered dose was eliminated in the urine, with 20% of that dose being the unchanged drug. About 20% of the dose was eliminated in the feces, with 15% of that dose being an unchanged drug. Following intravenous administration, the volume of distribution was 76 L, indicating distribution into tissues. The total body clearance of baricitinib was 8.9 L/h in patients with rheumatoid arthritis. The total body clearance and half-life of baricitinib was 14.2 L/h in intubated patients with COVID-19 who received baricitinib via nasogastric (NG) or orogastric (OG) tube. Metabolism / Metabolites Baricitinib is metabolized by CYP3A4. Approximately 6% of the orally administered dose was identified as metabolites in urine and feces; however, no metabolites of baricitinib were quantifiable in plasma. Biological Half-Life The elimination half-life in patients with rheumatoid arthritis is approximately 12 hours. The elimination half-life was 10.8 hours in intubated patients with COVID-19 who received baricitinib via nasogastric (NG) or orogastric (OG) tube. Oral bioavailability in rats/mice (from [1]): - Rats (male Sprague-Dawley, 250–300 g, n=4/group): - Oral 10 mg/kg: Cmax=3.8 μg/mL, Tmax=1.5 h, t1/2=4.6 h, AUC0-24h=22.3 μg·h/mL; - IV 2 mg/kg: Cmax=9.2 μg/mL, t1/2=4.2 h, AUC0-∞=5.7 μg·h/mL; - Oral bioavailability=79%; - Mice (male C57BL/6, 20–22 g, n=3/group): - Oral 10 mg/kg: Cmax=5.1 μg/mL, Tmax=1.0 h, t1/2=3.8 h, AUC0-24h=18.7 μg·h/mL [1] - Plasma protein binding (from [1]): - Human plasma: 97% (equilibrium dialysis, 37°C, 4 h); - Rat plasma: 96%; Mouse plasma: 95% [1] - Tissue distribution in CIA mice (from [1]): - Oral 10 mg/kg, 2 h post-dose: - Synovial tissue concentration=3.5 μg/g (1.1-fold of plasma concentration, 3.2 μg/mL); - Liver concentration=4.8 μg/g, spleen concentration=4.2 μg/g [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In the large prelicensure clinical trials in rheumatoid arthritis, serum aminotransferase elevations occurred in up to 17% of baricitinib treated subjects compared to 11% in placebo recipients. The elevations were typically mild and transient and values above 3 times the upper limit of normal (ULN) occurred in 1% to 2% of patients. The elevations occasionally led to early discontinuations, but more often resolved even without dose adjustment. In prelicensure studies in rheumatoid arthritis, alopecia areata and other rheumatic and immune-mediated disorders, there were no instances of clinically apparent liver injury attributed to baricitinib. Since approval and more wide scale availability of baricitinib, there have been no published reports of hepatotoxicity associated with its use. Use of baricitinib in combination with remdesivir for severe COVID-19 pneumonia has been reported but with little information on its potential for causing liver injury. Patients with severe SARS-CoV-2 infection frequently have elevated serum aminotransferase levels and occasionally are jaundiced. Furthermore, remdesivir has been linked to serum aminotransferase elevations during therapy that are generally mild-to-moderate in severity and resolve rapidly once the drug is stopped. Whether baricitinib increases the risk of liver injury during COVID-19 has yet to be shown, but hepatotoxicity was not a prominent feature in these early studies of its use in patients with severe COVID-19. Finally, baricitinib is an immune modulatory agent and has the potential of causing reactivation of viral infections including hepatitis B. In clinical trials, patients with HBsAg in serum were excluded from enrollment but patients with anti-HBc without HBsAg were allowed. While routine monitoring for reactivation was not performed on all patients, at least 15% of anti-HBc positive persons with rheumatoid arthritis treated with baricitinib developed virologic evidence of reactivation marked by de novo appearance of low levels of HBV DNA in serum. In all cases, the period of viremia was brief and not associated with serum aminotransferase elevations or jaundice. Thus, baricitinib appears to be capable of causing HBV reactivation but it is generally subclinical. Furthermore, the short courses of baricitinib used in the treatment of severe COVID-19 have not been linked to episodes of HBV reactivation. Likelihood score: E* (unlikely to be a cause of idiosyncratic clinically apparent liver injury but has the potential to cause reactivation of hepatitis B). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the use of baricitinib during breastfeeding. Most sources recommend that mothers not breastfeed while taking baricitinib. An alternate drug is preferred, especially while nursing a newborn or preterm infant. The manufacturer recommends that women avoid nursing during therapy and for 4 days after the last dose. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Baricitinib is approximately 50% bound to plasma proteins and 45% bound to serum proteins. Rat 28-day repeat-dose toxicity (from [1]): - Male/female Sprague-Dawley rats (n=4/sex/group), oral doses: 1 mg/kg, 10 mg/kg, 30 mg/kg daily. - No mortality or overt toxicity (lethargy, diarrhea); NOAEL=30 mg/kg. - 30 mg/kg group: Mild, reversible thrombocytopenia (15% reduction vs. control), no histopathological changes in liver/kidney/synovium; serum ALT/AST/creatinine/BUN normal [1] - Mouse acute toxicity (from [1]): - Male C57BL/6 mice, single oral dose up to 200 mg/kg: No mortality, body weight change ≤5% [1] - Clinical safety (from [2]): - 2 patients, Baricitinib 4 mg/day oral for 24/20 weeks: No severe adverse events; 1 patient had mild upper respiratory infection (self-resolved); no abnormal liver/kidney function (ALT/AST/creatinine) [2] |
| 参考文献 |
|
| 其他信息 |
Pharmacodynamics
Baricitinib is a disease-modifying antirheumatic drug (DMARD) used to ameliorate symptoms and slow down the progression of rheumatoid arthritis. In animal models of inflammatory arthritis, baricitinib was shown to have significant anti-inflammatory effects but also led to the preservation of cartilage and bone, with no detectable suppression of humoral immunity or adverse hematologic effects. Baricitinib decreased the levels of immunoglobulins and serum C-reactive protein in patients with rheumatoid arthritis. Mechanism of action (from [1,2]): 1. In arthritis: Baricitinib inhibits JAK1/JAK2, blocking cytokine (IL-6, TNF-α, IFN-γ) signaling via STAT phosphorylation suppression, reducing synovial inflammation and joint damage [1]; 2. In alopecia areata: Inhibits JAK1/JAK2, reducing IFN-γ/IL-15/IL-22 production by follicular Th1/Th22 cells, alleviating perifollicular inflammation and restoring hair follicle growth [2] - Therapeutic potential (from [1,2]): - Preclinical data supports use in rheumatoid arthritis (RA) [1]; - Clinical case data supports potential for alopecia areata (refractory cases) [2] - Drug class (from [1]): Baricitinib belongs to the pyrrolo[2,3-d]pyrimidine class of JAK inhibitors, optimized for JAK1/JAK2 selectivity and oral bioavailability [1] |
| 分子式 |
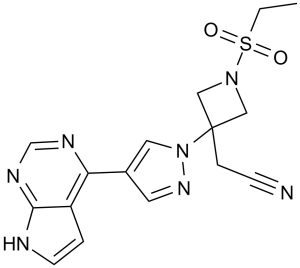
C16H17N7O2S
|
|
|---|---|---|
| 分子量 |
371.42
|
|
| 精确质量 |
371.116
|
|
| 元素分析 |
C, 51.74; H, 4.61; N, 26.40; O, 8.62; S, 8.63
|
|
| CAS号 |
1187594-09-7
|
|
| 相关CAS号 |
Baricitinib phosphate;1187595-84-1;Baricitinib-d5;1564241-79-7;Baricitinib-d3;1564242-30-3
|
|
| PubChem CID |
44205240
|
|
| 外观&性状 |
Typically exists as white to gray solids at room temperature
|
|
| 密度 |
1.6±0.1 g/cm3
|
|
| 沸点 |
707.2±70.0 °C at 760 mmHg
|
|
| 闪点 |
381.5±35.7 °C
|
|
| 蒸汽压 |
0.0±2.3 mmHg at 25°C
|
|
| 折射率 |
1.763
|
|
| LogP |
-0.06
|
|
| tPSA |
128.94
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
7
|
|
| 可旋转键数目(RBC) |
5
|
|
| 重原子数目 |
26
|
|
| 分子复杂度/Complexity |
678
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
S(C([H])([H])C([H])([H])[H])(N1C([H])([H])C(C([H])([H])C#N)(C1([H])[H])N1C([H])=C(C2=C3C([H])=C([H])N([H])C3=NC([H])=N2)C([H])=N1)(=O)=O
|
|
| InChi Key |
XUZMWHLSFXCVMG-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C16H17N7O2S/c1-2-26(24,25)22-9-16(10-22,4-5-17)23-8-12(7-21-23)14-13-3-6-18-15(13)20-11-19-14/h3,6-8,11H,2,4,9-10H2,1H3,(H,18,19,20)
|
|
| 化学名 |
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.73 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (6.73 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (6.73 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 0.5% CMC+0.25% Tween 80:30mg/mL 配方 5 中的溶解度: 2.5 mg/mL (6.73 mM) in 0.5% Methylcellulose/saline water (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6924 mL | 13.4618 mL | 26.9237 mL | |
| 5 mM | 0.5385 mL | 2.6924 mL | 5.3847 mL | |
| 10 mM | 0.2692 mL | 1.3462 mL | 2.6924 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04901325 | Recruiting | Drug: Baricitinib | RPyoderma Gangrenosum Skin Diseases |
Oregon Health and Science University | October 2023 | Phase 2 |
| NCT05852171 | Recruiting | Drug: Baricitinib | Mastitis Chronic Idiopathic Granulomatous Mastitis |
First Affiliated Hospital of Zhejiang University |
January 1, 2023 | Phase 2 |
| NCT05074420 | Recruiting | Drug: Baricitinib | Covid19 Corona Virus Infection |
Eli Lilly and Company | December 21, 2021 | Phase 3 |
| NCT06240351 | Not yet recruiting | Drug: Baricitinib 4 MG Oral Tablet | Frontal Fibrosing Alopecia | University of Alabama at Birmingham |
June 1, 2024 | Phase 4 |
Cellular activity of INCB028050.J Immunol.2010 May 1;184(9):5298-307. |
Anti-inflammatory and DMARD activity of once daily INCB028050 in rats with established disease in the adjuvant arthritis model.J Immunol.2010 May 1;184(9):5298-307. |
Suppression of delayed-type hypersensitivity by INCB028050.J Immunol.2010 May 1;184(9):5298-307. |
INCB028050 is efficacious and well tolerated independently of effects on humoral immunity.J Immunol.2010 May 1;184(9):5298-307. |
INCB028050 improves clinical and histologic signs of disease in the murine CIA model. |