| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
BB-Cl-amidine 抑制小鼠骨髓中性粒细胞的NET形成。用 12.5 µM BB-Cl-amidine 预处理中性粒细胞可显著减少PMA诱导的NET形成。[1]
BB-Cl-amidine 不抑制PMA刺激的小鼠中性粒细胞产生过氧化氢 (H2O2),表明它不阻断NADPH氧化酶通路。[1] 在使用人骨肉瘤 (U2OS) 细胞的细胞活力实验中,BB-Cl-amidine 的EC50为 8.8 ± 0.6 µM。其细胞效力比相关抑制剂Cl-amidine (EC50 > 200 µM) 高20倍以上。[1] BB-Cl-amidine 在体外可诱导新鲜分离的小鼠脾细胞凋亡。[1] |
|---|---|
| 体内研究 (In Vivo) |
在 MRL/lpr 动物中,用 BB-Cl-脒治疗在一定程度上减少了脾肿大,但用 PAD 抑制剂治疗往往会增加循环中的抗 NET 抗体的量。然而,体重和总 IgG 水平不受任何 PAD 抑制剂的影响。 Cl-脒和BB-Cl-脒疗法实际上极大地增强了内皮依赖性血管舒张。此外,BB-Cl-脒组中存在明显的IRG下调趋势。 Cl-脒或 BB-Cl-脒疗法可以显着减少口腔脱发,并且在许多情况下可以完全阻止脱发[1]。
在狼疮易感的MRL/lpr小鼠中,从8周龄到14周龄,每日皮下注射BB-Cl-amidine (1 mg/kg/天),与溶剂对照组相比,显著降低了骨髓中性粒细胞的离体自发性和PMA刺激的NET形成。[1] BB-Cl-amidine 治疗显著改善了从MRL/lpr小鼠分离的主动脉环的内皮依赖性血管舒张功能(血管功能的一项指标)。[1] BB-Cl-amidine 治疗下调了MRL/lpr小鼠骨髓中多个I型干扰素调节基因(如Mx1, Ifit1, Ifi44)的表达,并减少了肾脏中Mx1蛋白的表达。[1] BB-Cl-amidine 治疗减少了MRL/lpr小鼠肾小球中的免疫复合物沉积(IgG和C3),减轻了间质性炎症,并显著降低了尿白蛋白与肌酐比值(蛋白尿)。[1] BB-Cl-amidine 治疗显著改善了MRL/lpr小鼠的皮肤病变,预防或减轻了口鼻部脱毛。这种改善与皮肤中真皮层NETs(MPO-DNA复合物)和Mx1阳性细胞的减少相关。[1] BB-Cl-amidine 治疗轻微减轻了MRL/lpr小鼠的脾脏重量(脾肿大)。[1] BB-Cl-amidine 治疗没有显著改变MRL/lpr小鼠的循环中性粒细胞、淋巴细胞、血小板数量、血细胞比容、总IgG水平或体重。治疗后,循环抗dsDNA和抗CRAMP(一种NET成分)自身抗体水平有升高趋势。[1] |
| 酶活实验 |
评估了BB-Cl-amidine 对PAD酶 (PADs 1-4) 的效力和选择性。抑制效力以 kinact/KI 表示,这是不可逆抑制剂的最佳衡量指标。文献中以图表形式展示了BB-Cl-amidine 对PADs 1-4的 kinact/KI 值,与Cl-amidine相似。 [1]
|
| 细胞实验 |
进行了细胞生长抑制实验以评估BB-Cl-amidine 的细胞效力。将人骨肉瘤 (U2OS) 细胞用不同浓度的抑制剂处理72小时。然后使用XTT法(一种基于代谢活跃细胞还原四唑盐的比色法)测量细胞活力。根据剂量反应曲线计算EC50值。[1]
对新鲜分离的小鼠脾细胞进行了凋亡检测。用BB-Cl-amidine 处理细胞,并评估其诱导凋亡的能力。主文中未详细说明凋亡检测的具体方法(例如Annexin V/PI染色)。[1] |
| 动物实验 |
Animal/Disease Models: MRL/lpr mouse[1].
Doses: 1 mg/kg. Route of Administration: Administer subcutaneously (sc) (sc) daily from 8 to 14 weeks of age. Experimental Results: Endothelium-dependent vasodilation was Dramatically improved and demonstrated a strong trend of IRG downregulation. For in vivo efficacy studies in the MRL/lpr lupus model, BB-Cl-amidine was dissolved in a vehicle consisting of 25% DMSO in PBS. [1] Lupus-prone MRL/lpr mice (starting at 8 weeks of age) were treated daily with BB-Cl-amidine at a dose of 1 mg/kg via subcutaneous injection. Treatment continued for 6 weeks until the mice were euthanized at 14 weeks of age. Vehicle-treated mice received the 25% DMSO/PBS solution. [1] For pharmacokinetic studies, C57BL/6 mice were injected with BB-Cl-amidine at a dose of 1 mg/kg. Plasma was collected at various time points for analysis. [1] |
| 药代性质 (ADME/PK) |
Following a single injection in C57BL/6 mice, BB-Cl-amidine had a significantly longer in vivo plasma half-life (approximately 1.75 hours) compared to Cl-amidine (~15 minutes). [1]
In a murine hepatic microsome stability assay, BB-Cl-amidine showed similar stability to Cl-amidine, indicating comparable resistance to metabolic degradation by liver enzymes. [1] The enhanced cellular activity of BB-Cl-amidine compared to Cl-amidine is attributed to increased cellular uptake, likely due to its greater hydrophobicity. [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
In vitro, BB-Cl-amidine induced apoptosis in freshly isolated splenocytes. [1]
In vivo, treatment of MRL/lpr mice with BB-Cl-amidine for 6 weeks did not cause significant changes in circulating blood cell counts (neutrophils, lymphocytes, platelets), hematocrit, or body weight. It did not significantly alter splenic dendritic cell or T cell populations. [1] |
| 参考文献 | |
| 其他信息 |
BB-Cl-amidine is a novel, second-generation PAD inhibitor synthesized as a C-terminal bioisostere of Cl-amidine. Its structure incorporates a benzimidazole group at the C-terminus to prevent proteolysis and a biphenyl moiety at the N-terminus to increase hydrophobicity and enhance cellular uptake. [1]
The primary mechanism of action is the inhibition of PAD4-mediated histone citrullination, which is essential for NET formation. By inhibiting NETs, BB-Cl-amidine reduces the exposure of immunostimulatory molecules (like DNA and antimicrobial peptides), thereby decreasing type I interferon production and subsequent inflammation and tissue damage. [1] This study demonstrates that PAD inhibition with BB-Cl-amidine is protective in the MRL/lpr lupus model, improving vascular, kidney, and skin disease. This contrasts with the exacerbation of lupus observed with genetic inhibition of NET formation via NOX2 deletion in the same model, highlighting that the strategy for NET inhibition is critical. [1] The study suggests that PAD inhibition, by targeting a downstream step in NETosis (citrullination) while preserving upstream neutrophil functions like ROS production, may be a safer therapeutic strategy compared to broadly disrupting NADPH oxidase activity. [1] |
| 分子式 |
C₂₆H₂₆CLN₅O
|
|---|---|
| 分子量 |
459.97
|
| 精确质量 |
459.182
|
| 元素分析 |
C, 67.89; H, 5.70; Cl, 7.71; N, 15.23; O, 3.48
|
| CAS号 |
1802637-39-3
|
| 相关CAS号 |
BB-Cl-Amidine hydrochloride;2436747-41-8
|
| PubChem CID |
129021946
|
| 外观&性状 |
Solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 折射率 |
1.655
|
| LogP |
3.86
|
| tPSA |
96.2
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
9
|
| 重原子数目 |
33
|
| 分子复杂度/Complexity |
645
|
| 定义原子立体中心数目 |
1
|
| SMILES |
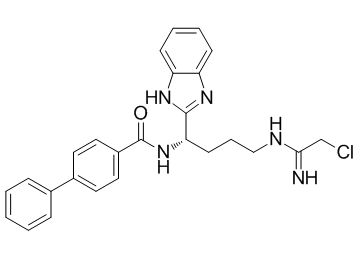
ClC/C(/N)=N\CCC[C@@H](C1=NC2C=CC=CC=2N1)NC(C1C=CC(C2C=CC=CC=2)=CC=1)=O
|
| InChi Key |
YDOAWJHYHGBQFI-QHCPKHFHSA-N
|
| InChi Code |
InChI=1S/C26H26ClN5O/c27-17-24(28)29-16-6-11-23(25-30-21-9-4-5-10-22(21)31-25)32-26(33)20-14-12-19(13-15-20)18-7-2-1-3-8-18/h1-5,7-10,12-15,23H,6,11,16-17H2,(H2,28,29)(H,30,31)(H,32,33)/t23-/m0/s1
|
| 化学名 |
N-[(1S)-1-(1H-benzimidazol-2-yl)-4-[(2-chloro-1-iminoethyl)amino]butyl]-[1,1'-biphenyl]-4-carboxamide
|
| 别名 |
BB-Cl-Amidine BB-CLA
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~125 mg/mL (~271.76 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (4.52 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (4.52 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (4.52 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1741 mL | 10.8703 mL | 21.7405 mL | |
| 5 mM | 0.4348 mL | 2.1741 mL | 4.3481 mL | |
| 10 mM | 0.2174 mL | 1.0870 mL | 2.1741 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。