| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|

| 靶点 |
Mtb F1FO-ATP synthase
|
|---|---|
| 体外研究 (In Vitro) |
Bedaquiline 可抑制 TDR 结核分枝杆菌菌株的生长,其 MIC 值范围为 0.125 至 0.5 mg/L[1]。贝达喹啉的 MIC50 和 MIC90 值分别为 0.03 和 16 mg/L,在缓慢生长的分枝杆菌 (SGM) 中对鸟分枝杆菌具有最强的活性。在快速生长的分枝杆菌 (RGM) 中,脓肿分枝杆菌亚种的 MIC50 和 MIC90 值分别为 0.13 和 >16 mg/L,脓肿分枝杆菌 (M.脓肿) 和脓肿分枝杆菌亚种。马西林 (M. Massiliense) 似乎比偶发分枝杆菌更容易受到贝达喹啉的影响。贝达喹啉对 NTM 物种具有中等的体外活性[2]。贝达喹啉对结核分枝杆菌,包括耐多药结核分枝杆菌的体外活性非常好[3]。
|
| 体内研究 (In Vivo) |
BDQ在斑马鱼脓肿分枝杆菌感染模型中非常有效。值得注意的是,很短的处理时间就足以保护受感染的幼虫免受脓肿分枝杆菌诱导的杀死。脓肿和脊髓数量的减少证实了这一点,脓肿和脊髓被认为是受感染斑马鱼的主要病理生理体征。[7]
|
| 酶活实验 |
胞内ATP定量
使用96孔平板测定细胞内ATP水平,如先前对结核分枝杆菌所述。脓肿分枝杆菌暴露于BDQ或阿米卡星(阴性对照),并在32°C下孵育180分钟。将25微升脓肿分枝杆菌培养物与等体积的BacTiter-Glo试剂在96孔平底白色板中混合,并在黑暗中孵育5分钟。使用BioTek Cytation 3多模读取器检测发光,并使用GraphPad Prism 6软件绘制所获得的值。[7]
|
| 细胞实验 |
药物敏感性测试。[7]
根据CLSI指南,使用指数生长期含有5×106CFU/ml的接种物,基于CaMHB中肉汤微量稀释法测定MIC。将细菌(100μl)接种在96孔板中,并将最高浓度的2μl药物添加到含有两倍体积的细菌悬浮液(200μl)的第一孔中。然后进行两次系列稀释,并在30°C下与药物孵育3-5天。通过目视检查和在560nm处的吸光度来记录MIC,以确认目视记录。实验分三次独立进行,一式三份。 时间杀伤分析。[7]
建立微量滴定板用于MIC测定。在暴露于不同药物浓度0、24、48、72和96小时后,对细菌悬浮液进行连续稀释。在30°C下培养4天后计数CFU。
|
| 动物实验 |
Assessment of BDQ efficacy in infected zebrafish. [7]
Rough M. abscessus CIP104536T (ATCC 19977T) carrying pTEC27 (plasmid 30182; Addgene) and expressing the red fluorescent protein tdTomato was prepared and microinjected in zebrafish embryos, according to procedures described earlier. Briefly, mid-log-phase cultures of M. abscessus expressing tdTomato were centrifuged, washed, and resuspended in phosphate-buffered saline (PBS) supplemented with 0.05% Tween 80 (PBS-T). Bacterial suspensions were then homogenized through a 26-gauge needle and sonicated, and the remaining clumps were allowed to settle down for 5 to 10 min. Bacteria were concentrated to an optical density at 600 nm (OD600) of 1 in PBS-T and injected intravenously (≈2 to 5 nl containing 50 to 300 CFU) into the caudal vein in 30-h-postfertilization (hpf) embryos previously dechorionated and anesthetized. To follow infection kinetics and embryo survival, infected larvae were transferred into 24-well plates (2 embryos/well) and incubated at 28.5°C. The CFU numbers in the inoculum were determined by injection of 2 nl of the bacterial suspension in sterile PBS-T and plating on 7H10 with 500 μg/ml hygromycin.
|
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Data from two women taking bedaquiline and one of their breastfed infants indicate that exposure of the infant to the drug via breastmilk is substantial, with one infant having a therapeutic serum level. The clinical consequences of this exposure are unknown. The drug could protect the infant from multidrug-resistant tuberculosis, or could result in adverse effects. If bedaquiline is required by the mother, it is not a reason to discontinue breastfeeding. Monitor breastfed infants for adverse reactions, such as inadequate weight gain, liver toxicity, nausea, arthralgia, headache, hemoptysis, and chest pain. ◉ Effects in Breastfed Infants A woman who was co-infected with HIV and rifampin-resistant tuberculosis took bedaquiline (dosage not stated) as part of her antituberculosis regimen, which consisted of pyrazinamide and other unnamed drugs. At the 1-month follow-up, the infant was small and not gaining weight well, but the mother was nauseated from her medication regimen and had also lost weight. Six months later after completion of the mother’s therapy, her infant’s weight was increasing, following the normal trajectory of the growth chart, and reaching her developmental milestones. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. |
| 参考文献 | |
| 其他信息 |
Bedaquiline fumarate is a fumarate salt prepared from equimolar amounts of bedaquiline and fumaric acid. It is used in combination therapy for the treatment of pulmonary multi-drug resistant tuberculosis by inhibition of ATP synthase, an enzyme essential for the replication of the mycobacteria. It has a role as an antitubercular agent and an ATP synthase inhibitor. It contains a bedaquiline(2+).
Bedaquiline Fumarate is the fumarate salt form of bedaquiline, an orally bioavailable diarylquinoline antimycobacterial agent, that can be used in the treatment of pulmonary multi-drug resistant tuberculosis (MDR-TB). Upon oral administration, bedaquiline specifically binds to subunit c of Mycobacterium tuberculosis (M. tuberculosis) adenosine 5'-triphosphate (ATP) synthase, thereby preventing ATP synthase activity. This inhibits ATP synthesis in M. tuberculosis, thereby blocking its energy metabolism and killing M. tuberculosis. See also: Bedaquiline (has active moiety). Drug Indication Sirturo is indicated for use as part of an appropriate combination regimen for pulmonary multidrug resistant tuberculosis (MDR TB) in adults and adolescent patients (12 years to less than 18 years of age and weighing at least 30 kg) when an effective treatment regimen cannot otherwise be composed for reasons of resistance or tolerability. Â Consideration should be given to official guidance on the appropriate use of antibacterial agents. Treatment of multi-drug-resistant tuberculosis |
| 分子式 |
C36H35BRN2O6
|
|---|---|
| 分子量 |
671.59
|
| 精确质量 |
670.167
|
| 元素分析 |
C, 64.38; H, 5.25; Br, 11.90; N, 4.17; O, 14.29
|
| CAS号 |
845533-86-0
|
| 相关CAS号 |
Bedaquiline;843663-66-1;(Rac)-Bedaquiline;654655-80-8
|
| PubChem CID |
24812732
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
6.842
|
| tPSA |
120.19
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
8
|
| 可旋转键数目(RBC) |
10
|
| 重原子数目 |
45
|
| 分子复杂度/Complexity |
834
|
| 定义原子立体中心数目 |
2
|
| SMILES |
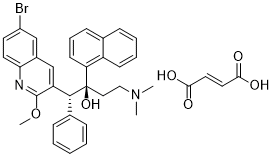
OC(/C=C/C(O)=O)=O.BrC1=CC=C(N=C(OC)C([C@H]([C@@](C2=CC=CC3=C2C=CC=C3)(O)CCN(C)C)C4=CC=CC=C4)=C5)C5=C1
|
| InChi Key |
ZLVSPMRFRHMMOY-WWCCMVHESA-N
|
| InChi Code |
InChI=1S/C32H31BrN2O2.C4H4O4/c1-35(2)19-18-32(36,28-15-9-13-22-10-7-8-14-26(22)28)30(23-11-5-4-6-12-23)27-21-24-20-25(33)16-17-29(24)34-31(27)37-3;5-3(6)1-2-4(7)8/h4-17,20-21,30,36H,18-19H2,1-3H3;1-2H,(H,5,6)(H,7,8)/b;2-1+/t30-,32-;/m1./s1
|
| 化学名 |
(1R,2S)-1-(6-Bromo-2-methoxy-3-quinolyl)-4-dimethylamino-2-(1-naphthyl)-1-phenyl-butan-2-ol fumarate
|
| 别名 |
R207910 fumarate; TMC-207 fumarate; R-207910; TMC 207; R 207910; TMC207 fumarate; Bedaquiline fumarate; trade name: Sirturo
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL ( ~148.9 mM )
Ethanol : ~4 mg/mL |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.75 mg/mL (4.09 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 27.5 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 2.75 mg/mL (4.09 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 27.5mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.75 mg/mL (4.09 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: ≥ 2.75 mg/mL (4.09 mM) (饱和度未知) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 5 中的溶解度: ≥ 2.75 mg/mL (4.09 mM) (饱和度未知) in 5% DMSO + 95% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 6 中的溶解度: 配方 1 中的溶解度: ≥ 2.8 mg/mL (4.1 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,您可以取100 μL 28 mg/mL DMSO 储备液加入到400 μL PEG300中,混匀(澄清溶液);然后再向上述溶液中加入50 μL Tween 80,混匀(澄清溶液);最后向上述溶液中加入450 μL 生理盐水,混匀(澄清溶液)。 生理盐水的配制:将0.9 g氯化钠溶解于100 mL ddH ₂ O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.8 mg/mL (4.1 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,如果要制备1 mL工作液,则可以取100 μL 28 mg/mL DMSO储备液并添加到900 μL 20% SBE-β-CD 生理盐水溶液,充分混合(澄清溶液)。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 3 中的溶解度: ≥ 2.8 mg/mL (4.1 mM) (饱和度未知) in 10% DMSO + 90% Corn oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,如果要制备1 mL工作液,则可以取100 μL 28 mg/mL DMSO储备液并添加到900 μL 玉米油,混合均匀(澄清溶液)。 配方 4 中的溶解度: ≥ 2.8 mg/mL (4.1 mM) (饱和度未知) in 5% DMSO + 40% PEG300 + 5% Tween80 + + 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 5 中的溶解度: ≥ 2.8 mg/mL (4.1 mM) (饱和度未知) in 5% DMSO + 95% (20% SBE-β-CD in saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.4890 mL | 7.4450 mL | 14.8900 mL | |
| 5 mM | 0.2978 mL | 1.4890 mL | 2.9780 mL | |
| 10 mM | 0.1489 mL | 0.7445 mL | 1.4890 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Distribution of MIC values for rapidly growing mycobacterial strains. The arrows represent the proposed ECOFF value for rapidly growing mycobacteria.Antimicrob Agents Chemother.2017 Apr 24;61(5). pii: e02627-16. |
|---|
Distribution of MIC values for slowly growing mycobacterial strains. The arrows represent the proposed ECOFF value for slowly growing mycobacteria.Antimicrob Agents Chemother.2017 Apr 24;61(5). pii: e02627-16. |