| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|

| 靶点 |
Mtb F1FO-ATP synthase
|
|---|---|
| 体外研究 (In Vitro) |
贝达喹啉可抑制 TDR 结核分枝杆菌的生长,其 MIC 值范围为 0.125 至 0.5 mg/L[2]。
贝达喹啉的 MIC50 和 MIC90 值分别为 0.03 和 16 mg/L,活性最强对抗缓慢生长的分枝杆菌(SGM)中的鸟分枝杆菌。脓肿分枝杆菌亚种的 MIC50 和 MIC90 值分别为 0.13 和 >16 mg/L。脓肿(M.脓肿)和脓肿分枝杆菌亚种。在快速生长的分枝杆菌 (RGM) 中,massiliense (M. Massiliense) 似乎比偶然分枝杆菌更容易受到贝达喹啉的影响。还证明了贝达喹啉对 NTM 物种具有中等的体外活性[3]。 贝达喹啉对结核分枝杆菌(包括耐多药结核分枝杆菌)的体外活性非常好[4]。 |
| 体内研究 (In Vivo) |
BDQ在斑马鱼脓肿分枝杆菌感染模型中非常有效。值得注意的是,很短的处理时间就足以保护受感染的幼虫免受脓肿分枝杆菌诱导的杀死。脓肿和脊髓数量的减少证实了这一点,脓肿和脊髓被认为是受感染斑马鱼的主要病理生理体征。[7]
|
| 酶活实验 |
细胞内ATP定量
使用96孔平板测定细胞内ATP水平,如先前对结核分枝杆菌所述。脓肿分枝杆菌暴露于BDQ或阿米卡星(阴性对照),并在32°C下孵育180分钟。将25微升脓肿分枝杆菌培养物与等体积的BacTiter-Glo试剂在96孔平底白色板中混合,并在黑暗中孵育5分钟。使用BioTek Cytation 3多模读取器检测发光,并使用GraphPad Prism 6软件绘制所获得的值。[7] |
| 细胞实验 |
药物敏感性测试。[7]
根据CLSI指南,使用指数生长期含有5×106CFU/ml的接种物,基于CaMHB中肉汤微量稀释法测定MIC。将细菌(100μl)接种在96孔板中,并将最高浓度的2μl药物添加到含有两倍体积的细菌悬浮液(200μl)的第一孔中。然后进行两次系列稀释,并在30°C下与药物孵育3-5天。通过目视检查和在560nm处的吸光度来记录MIC,以确认目视记录。实验分三次独立进行,一式三份。 时间杀伤分析。[7] 建立微量滴定板用于MIC测定。在暴露于不同药物浓度0、24、48、72和96小时后,对细菌悬浮液进行连续稀释。在30°C下培养4天后计数CFU。 |
| 动物实验 |
Assessment of BDQ efficacy in infected zebrafish. [7]
Rough M. abscessus CIP104536T (ATCC 19977T) carrying pTEC27 (plasmid 30182; Addgene) and expressing the red fluorescent protein tdTomato was prepared and microinjected in zebrafish embryos, according to procedures described earlier. Briefly, mid-log-phase cultures of M. abscessus expressing tdTomato were centrifuged, washed, and resuspended in phosphate-buffered saline (PBS) supplemented with 0.05% Tween 80 (PBS-T). Bacterial suspensions were then homogenized through a 26-gauge needle and sonicated, and the remaining clumps were allowed to settle down for 5 to 10 min. Bacteria were concentrated to an optical density at 600 nm (OD600) of 1 in PBS-T and injected intravenously (≈2 to 5 nl containing 50 to 300 CFU) into the caudal vein in 30-h-postfertilization (hpf) embryos previously dechorionated and anesthetized. To follow infection kinetics and embryo survival, infected larvae were transferred into 24-well plates (2 embryos/well) and incubated at 28.5°C. The CFU numbers in the inoculum were determined by injection of 2 nl of the bacterial suspension in sterile PBS-T and plating on 7H10 with 500 μg/ml hygromycin. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
After the recommended dosing regimen of bedaquiline (400 mg for 2 weeks followed by 200 mg three times per week for 22 weeks), the Cmax and AUC24h were calculated to be 1.659 μg/ml and 25.863 μg.h/ml respectively. After a single oral dose administration of bedaquiline, maximum plasma concentrations (Cmax) are typically achieved at approximately 5 hours post-dose. Cmax and the area under the plasma concentration-time curve (AUC) increased proportionally up to 700 mg (1.75 times the 400 mg loading dose). Administration of bedaquiline with a standard meal containing approximately 22 grams of fat (558 total Kcal) increased the relative bioavailability by approximately 2-fold compared to administration under fasted conditions. Bedaquiline should be taken with food to enhance its oral bioavailability. After reaching Cmax, bedaquiline concentrations decline tri-exponentially. Based on preclinical studies, bedaquiline is mainly excreted in feces. The urinary excretion of unchanged bedaquiline was less than or equal to 0.001% of the dose in clinical studies, indicating that renal clearance of unchanged drug is insignificant. The volume of distribution in the central compartment is estimated to be approximately 164 Liters. Bedaquiline has a low apparent clearance of approximately 2.78 L/h. Bedaquiline is a novel agent for the treatment of pulmonary multidrug-resistant Mycobacterium tuberculosis infections, in combination with other agents. The objective of this study was to develop a population pharmacokinetic (PK) model for bedaquiline to describe the concentration-time data from phase I and II studies in healthy subjects and patients with drug-susceptible or multidrug-resistant tuberculosis (TB). A total of 5,222 PK observations from 480 subjects were used in a nonlinear mixed-effects modeling approach. The PK was described with a 4-compartment disposition model with dual zero-order input (to capture dual peaks observed during absorption) and long terminal half-life (t1/2). The model included between-subject variability on apparent clearance (CL/F), apparent central volume of distribution (Vc/F), the fraction of dose via the first input, and bioavailability (F). Bedaquiline was widely distributed, with apparent volume at steady state of >10,000 liters and low clearance. The long terminal t1/2 was likely due to redistribution from the tissue compartments. The final covariate model adequately described the data and had good simulation characteristics. The CL/F was found to be 52.0% higher for subjects of black race than that for subjects of other races, and Vc/F was 15.7% lower for females than that for males, although their effects on bedaquiline exposure were not considered to be clinically relevant. Small differences in F and CL/F were observed between the studies. The residual unexplained variability was 20.6% and was higher (27.7%) for long-term phase II studies. Bedaquiline is distributed into milk in rats; it is not known whether the drug is distributed into human milk. The plasma protein binding of bedaquiline is > 99.9%. The volume of distribution in the central compartment is estimated to be approximately 164 L. After oral administration bedaquiline maximum plasma concentrations (Cmax) are typically achieved at approximately 5 hours post-dose. Cmax and the area under the plasma concentration-time curve (AUC) increased proportionally up to the highest doses studied in healthy volunteers (700 mg single-dose and once daily 400 multiple doses). Administration of bedaquiline with a standard meal containing approximately 22 grams of fat (558 total Kcal) increased the relative bioavailability by about 2-fold compared to administration under fasted conditions. Therefore, bedaquiline should be taken with food to enhance its oral bioavailability. For more Absorption, Distribution and Excretion (Complete) data for Bedaquiline (10 total), please visit the HSDB record page. Metabolism / Metabolites CYP3A4 was the major CYP isoenzyme involved in the in vitro metabolism of bedaquiline and the formation of the N-monodesmethyl metabolite (M2). CYP3A4 was the major CYP isoenzyme involved in vitro in the metabolism of bedaquiline and the formation of the N-monodesmethyl metabolite (M2), which is 4 to 6-times less active in terms of antimycobacterial potency. Based on preclinical studies, bedaquiline is mainly eliminated in feces. The urinary excretion of unchanged bedaquiline was < 0.001% of the dose in clinical studies, indicating that renal clearance of unchanged drug is insignificant. After reaching Cmax, bedaquiline concentrations decline tri-exponentially. The mean terminal elimination half-life of bedaquiline and the N-monodesmethyl metabolite (M2) is approximately 5.5 months. This long terminal elimination phase likely reflects slow release of bedaquiline and M2 from peripheral tissues. After a single dose the mean AUC0-24 hr of the major metabolite M2 was 2 to 7-fold higher than AUC0-24 hr of bedaquiline in mice and was generally similar to 2-fold lower in rats and dogs. Bedaquiline is a recently approved drug for the treatment of multidrug-resistant tuberculosis. Adverse cardiac and hepatic drug reactions to bedaquiline have been noted in clinical practice. The current study investigated bedaquiline metabolism in human hepatocytes using a metabolomic approach. Bedaquiline N-demethylation via CYP3A4 was confirmed as the major pathway in bedaquiline metabolism. In addition to CYP3A4, we found that both CYP2C8 and CYP2C19 contributed to bedaquiline N-demethylation. The Km values of CYP2C8, CYP2C19, and CYP3A4 in bedaquiline N-demethylation were 13.1, 21.3, and 8.5 uM, respectively. We also identified a novel metabolic pathway of bedaquiline that produced an aldehyde intermediate. In summary, this study extended our knowledge of bedaquiline metabolism, which can be applied to predict and prevent drug-drug interactions and adverse drug reactions associated with bedaquiline. No chiral conversion of bedaquiline occurred in vivo after administration of bedaquiline to mice, rats, dogs, monkeys and humans. In hepatocytes and subcellular fractions from preclinical species and humans, the in vitro metabolism of (14)C-bedaquiline was via Phase I reactions and the most important pathway was N-demethylation to M2, which was followed by a second N-demethylation to M3, oxidation and epoxidation. M2 was the major circulating metabolite in all preclinical species as determined by radioactivity profiling and LC-MS/MS in the animals. No mass balance study with radiolabelled bedaquiline has been conducted in humans. It can therefore not be excluded that additional undetected metabolites may be formed in humans that are not formed in the animal species. M2-AUC0-24 hr plasma levels were generally comparable to 2-fold lower than those of bedaquiline in rats and dogs upon repeated administration of bedaquiline, and 3.5- to 4.5-fold lower in human subjects with MDR-TB. In addition to M2 and M3, a hydroxylated derivative of M2 (M20) and a dihydrodiol derivative of M2 (M11), were detected in human plasma. These two metabolites were also found in rats and dogs at similar relative concentrations. Biological Half-Life The mean terminal elimination half-life of bedaquiline and the N-monodesmethyl metabolite (M2) is approximately 5.5 months. This long terminal elimination phase likely reflects the slow release of bedaquiline and M2 from peripheral tissues. The plasma concentration-time profiles of bedaquiline showed a multi-phasic decline with a long terminal elimination half life ranging from 2 to 3 days in mice, 3 to 5 days in male rats, 6 to 9 days in female rats and monkeys and up to 50 days in dogs. The mean terminal elimination half-life of bedaquiline and the N-monodesmethyl metabolite (M2) is approximately 5.5 months. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Bedaquiline is a white solid. It is used as a antitubercular medication. HUMAN EXPOSURE AND TOXICITY: An increased risk of death was observed in patients receiving bedaquiline in a placebo-controlled clinical trial. In this study, there were 9 deaths in bedaquiline-treated patients; one death occurred during the 24 weeks of bedaquiline therapy and the median time to death for the other 8 patients was 329 days after the last dose of bedaquiline. Five of the 9 deaths in bedaquiline-treated patients and both deaths in placebo-treated patients were related to tuberculosis. The explanation for the imbalance in deaths in this study is not known; no correlation was demonstrated between death and sputum culture conversion, relapse, susceptibility to other antituberculosis drugs, HIV status, or disease severity. Bedaquiline and the M2 metabolite are cationic amphiphilic substances and induce phospholipidosis. The cells of the monocytic phagocytic system are affected in all species. Data from in vitro studies using human monocyte cell-line indicated that the phospholipidogenic potential was highest for the M2 metabolite followed by M3 and the parent compound. ANIMAL STUDIES: In mouse and rat, single oral doses of 800 mg/kg produced lethality preceded by signs of general toxicity. Mortalities in mouse and dog after single and repeated doses were principally attributed to skeletal muscle/myocardial degeneration and/or pancreatitis. Bedaquiline was not carcinogenic in rats up to the maximum tolerated dose of 10 mg/kg/day. In embryofetal toxicity studies conducted in rat and rabbit bedaquiline appeared to have no adverse effects on the embryonal development and the incidence of variations and malformations in fetuses in bedaquiline groups were within normal ranges. Exposure to bedaquiline and the M2 metabolite in rat at the high dose was considerable (up to 6-7 times higher compared with expected human exposure), while in rabbit a maximum exposure ratio of 2 were achieved. However, in rabbit the high dose of 100 mg/kg caused deaths, one abortion and increases in pre and postimplantation losses. Bedaquiline had no effect on fertility in females up to the highest dose tested, 24 mg/kg. Male fertility appeared to be decreased with a NOAEL of 5 mg/kg. No mutagenic or clastogenic effects were detected in the in vitro non-mammalian reverse mutation (Ames) test, in vitro mammalian (mouse lymphoma) forward mutation assay and an in vivo mouse bone marrow micronucleus assay. Hepatotoxicity Liver test abnormalities occur in 8% to 12% of patients treated with multiple drug regimens that include bedaquiline. These abnormalities are usually asymptomatic, mild-to-moderate in severity and self-limited in duration. In many instances, it is difficult to determine which of the antituberculosis medications accounts for the abnormalities, but monitoring of liver tests at monthly intervals is recommended during bedaquiline therapy. Clinically apparent liver injury has been reported with bedaquiline therapy, but the clinical features, course and outcome of these cases has not been described. At least three deaths from end stage liver disease have been described in patients taking bedaquiline, but the attribution of the hepatic failure to bedaquiline has been questioned. The management of multidrug resistant tuberculosis is challenging and should be under the direction of physicians with expertise in tuberculosis therapy. Likelihood score: E* (unproven but suspected cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Data from two women taking bedaquiline and one of their breastfed infants indicate that exposure of the infant to the drug via breastmilk is substantial, with one infant having a therapeutic serum level. The clinical consequences of this exposure are unknown. The drug could protect the infant from multidrug-resistant tuberculosis, or could result in adverse effects. If bedaquiline is required by the mother, it is not a reason to discontinue breastfeeding. Monitor breastfed infants for adverse reactions, such as inadequate weight gain, liver toxicity, nausea, arthralgia, headache, hemoptysis, and chest pain. ◉ Effects in Breastfed Infants A woman who was co-infected with HIV and rifampin-resistant tuberculosis took bedaquiline (dosage not stated) as part of her antituberculosis regimen, which consisted of pyrazinamide and other unnamed drugs. At the 1-month follow-up, the infant was small and not gaining weight well, but the mother was nauseated from her medication regimen and had also lost weight. Six months later after completion of the mother’s therapy, her infant’s weight was increasing, following the normal trajectory of the growth chart, and reaching her developmental milestones. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding The plasma protein binding of bedaquiline is greater than 99.9%. Interactions Pharmacologic interaction (increased risk of QT interval prolongation). Concomitant use with other drugs that prolong the QT interval (e.g., clofazimine, fluoroquinolones, macrolides) may result in additive or synergistic effects on the QT interval. Bedaquiline is metabolized primarily by cytochrome P-450 (CYP) isoenzyme 3A4. Concomitant use of bedaquiline with potent inhibitors of CYP3A4 (e.g., ketoconazole) may increase the area under the concentration-time curve (AUC) of bedaquiline and increase the risk of adverse effects associated with the drug. Concomitant use of bedaquiline and systemic drugs that are potent inhibitors of CYP3A4 for a duration longer than 14 consecutive days should be avoided, unless the benefits of concomitant use outweigh the risks. Patients receiving such concomitant therapy should be monitored for bedaquiline-related adverse effects. Concomitant use of bedaquiline with potent inducers of CYP3A4, including rifamycins (e.g., rifampin, rifapentine, rifabutin), may reduce the AUC of bedaquiline and decrease the therapeutic effects of the drug. Concomitant use of bedaquiline with rifamycins or other potent inducers of CYP3A4 should be avoided. Because concomitant use of bedaquiline and fluoroquinolones may increase the risk of QT interval prolongation, ECGs should be monitored closely during concomitant therapy. Because concomitant use of bedaquiline and macrolides may increase the risk of QT interval prolongation, ECGs should be monitored closely during concomitant therapy. For more Interactions (Complete) data for Bedaquiline (13 total), please visit the HSDB record page. |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
Antitubercular Agents Sirturo is a diarylquinoline antimycobacterial drug indicated as part of combination therapy in adults (= 18 years) with pulmonary multi-drug resistant tuberculosis (MDR-TB). Reserve Sirturo for use when an effective treatment regimen cannot otherwise be provided. Sirturo should be administered by directly observed therapy (DOT). This indication is based on analysis of time to sputum culture conversion from two controlled Phase 2 trials in patients with pulmonary MDR-TB. /Included in US product label/ The safety and efficacy of Sirturo for the treatment of latent infection due to Mycobacterium tuberculosis have not been established. The safety and efficacy of Sirturo for the treatment of drug-sensitive TB have not been established. In addition, there are no data on the treatment with Sirturo of extra-pulmonary TB (e.g., central nervous system). The safety and efficacy of Sirturo for the treatment of infections caused by non-tuberculous mycobacteria (NTM) have not been established. Therefore, use of SIRTURO in these settings is not recommended. For the first time in over 40 years, a new tuberculosis (TB) drug with a novel mechanism of action - bedaquiline - is available, and was granted accelerated approval by the United States Food and Drug Administration in December 2012. There is considerable interest in the potential of this drug to treat multidrug-resistant tuberculosis (MDR-TB). However, information about this new drug remains limited. It has only been through two Phase IIb trials for safety and efficacy. WHO is therefore issuing "interim policy guidance". This interim guidance provides advice on the inclusion of bedaquiline in the combination therapy of MDR-TB in accordance with the existing WHO Guidelines for the programmatic management of drug-resistant TB (2011 Update). The interim guidance lists five conditions that must be in place if bedaquiline is used to treat adults with MDR-TB: 1.Effective treatment and monitoring: Treatment must be closely monitored for effectiveness and safety, using sound treatment and management protocols approved by relevant national authorities. 2.Proper patient inclusion: Special caution is required when bedaquiline is used in people aged 65 and over, and in adults living with HIV. Use in pregnant women and children is not advised. 3.Informed consent: Patients must be fully aware of the potential benefits and harms of the new drug, and give documented informed consent before embarking on treatment. 4.Adherence to WHO recommendations: All principles on which WHO-recommended MDR-TB treatment regimens are based, must be followed, particularly the inclusion of four effective second-line drugs. In line with general principles of TB therapeutics, bedaquiline alone should not be introduced into a regimen in which the companion drugs are failing to show effectiveness. 5.Active pharmacovigilance and management of adverse events: Active pharmacovigilance measures must be in place to ensure early detection and proper management of adverse drug reactions and potential interactions with other drugs. WHO strongly recommends the acceleration of Phase III trials to generate a more comprehensive evidence base to inform future policy on bedaquiline. The Organization will review, revise, or update the interim guidance as additional information on efficacy and safety become available. Multidrug-resistant tuberculosis (MDR TB) is caused by Mycobacterium tuberculosis that is resistant to at least isoniazid and rifampin, the two most effective of the four first-line TB drugs (the other two drugs being ethambutol and pyrazinamide). MDR TB includes the subcategory of extensively drug-resistant TB (XDR TB), which is MDR TB with additional resistance to any fluoroquinolone and to at least one of three injectable anti-TB drugs (i.e., kanamycin, capreomycin, or amikacin). MDR TB is difficult to cure, requiring 18-24 months of treatment after sputum culture conversion with a regimen that consists of four to six medications with toxic side effects, and carries a mortality risk greater than that of drug-susceptible TB. Bedaquiline fumarate (Sirturo or bedaquiline) is an oral diarylquinoline. On December 28, 2012, on the basis of data from two Phase IIb trials (i.e., well-controlled trials to evaluate the efficacy and safety of drugs in patients with a disease or condition to be treated, diagnosed, or prevented), the Food and Drug Administration (FDA) approved use of bedaquiline under the provisions of the accelerated approval regulations for "serious or life-threatening illnesses" (21CFR314.500). ... This report provides provisional CDC guidelines for FDA-approved and unapproved, or off-label, uses of bedaquiline in certain populations, such as children, pregnant women, or persons with extrapulmonary MDR TB who were not included in the clinical trials for the drug. CDC's Division of TB Elimination developed these guidelines on the basis of expert opinion informed by data from systematic reviews and literature searches. This approach is different from the statutory standards that FDA uses when approving drugs and drug labeling. These guidelines are intended for health-care professionals who might use bedaquiline for the treatment of MDR TB for indicated and off-label uses. Aspects of these guidelines are not identical to current FDA-approved labeling for bedaquiline. Bedaquiline should be used with clinical expert consultation as part of combination therapy (minimum four-drug treatment regimen) and administered by direct observation to adults aged =18 years with a diagnosis of pulmonary MDR TB (Food and Drug Administration. Sirturo [bedaquiline] tablets label. ... Use of the drug also can be considered for individual patients in other categories (e.g., persons with extrapulmonary TB, children, pregnant women, or persons with HIV or other comorbid conditions) when treatment options are limited. However, further study is required before routine use of bedaquiline can be recommended in these populations. A registry for persons treated with bedaquiline is being implemented by ... to track patient outcomes, adverse reactions, laboratory testing results (e.g., diagnosis, drug susceptibility, and development of drug resistance), use of concomitant medications, and presence of other comorbid conditions. Suspected adverse reactions (i.e., any adverse event for which there is a reasonable possibility that the drug caused the adverse event) and serious adverse events (i.e., any adverse event that results in an outcome such as death, hospitalization, permanent disability, or a life-threatening situation) should be reported ... . Drug Warnings /BOXED WARNING/ WARNINGS: An increased risk of death was seen in the Sirturo treatment group (9/79, 11.4%) compared to the placebo treatment group (2/81, 2.5%) in one placebo-controlled trial. Only use Sirturo when an effective treatment regimen cannot otherwise be provided. QT prolongation can occur with Sirturo. Use with drugs that prolong the QT interval may cause additive QT prolongation. A higher incidence of adverse hepatic effects has been reported in patients receiving antituberculosis regimens containing bedaquiline compared with patients receiving regimens that did not contain the drug. Based on data from 2 clinical trials, reversible increases in serum aminotransferase concentrations to at least 3 times the upper limit of normal (ULN) were reported in 10.8 or 5.7% of patients receiving bedaquiline or placebo, respectively. Liver function tests (AST, ALT, alkaline phosphatase, bilirubin) should be monitored at baseline, monthly during treatment, and as needed. Patients also should be monitored for symptoms of hepatic dysfunction. If signs or symptoms of new or worsening liver dysfunction (e.g., clinically important elevation in serum aminotransferases and/or bilirubin, fatigue, anorexia, nausea, jaundice, dark urine, liver tenderness, hepatomegaly) develop, the patient should be promptly evaluated. If AST or ALT increase to greater than 3 times the ULN, liver function tests should be repeated within 48 hours. In addition, patients should be tested for viral hepatitis and other hepatotoxic drugs should be discontinued. Bedaquiline should be discontinued if elevated serum aminotransferase concentrations are accompanied by total bilirubin concentrations exceeding 2 times the ULN, serum aminotransferase concentrations exceed 8 times the ULN, or elevated aminotransferase concentrations persist for more than 2 weeks. Alcohol and other hepatotoxic drugs or herbal products should be avoided in patients receiving bedaquiline, especially in those with diminished hepatic reserve. Prolongation of the QT interval has occurred in patients receiving bedaquiline. Concomitant use of bedaquiline with other drugs associated with QT interval prolongation may result in additive or synergistic effects on the QT interval. Documented cases of torsades de pointes have not been reported to date in patients receiving bedaquiline. Safety and efficacy of bedaquiline have not been established in patients younger than 18 years of age. For more Drug Warnings (Complete) data for Bedaquiline (10 total), please visit the HSDB record page. Pharmacodynamics Bedaquiline is primarily subjected to oxidative metabolism leading to the formation of N-monodesmethyl metabolite (M2). M2 is not thought to contribute significantly to clinical efficacy given its lower average exposure (23% to 31%) in humans and lower antimycobacterial activity (4-fold to 6-fold lower) than the parent compound. However, M2 plasma concentrations appeared to correlate with QT prolongation. Bedaquiline inhibits mycobacterial TB at a minimal inhibitory concentration (MIC) from 0.002-0.06 μg/ml and with a MIC50 of 0.03 μg/ml. The proportion of naturally resistant bacteria is low, estimated to be in one strain over 107/108 bacteria. Bacteria that have smaller ATP stores (such as dormant, nonreplicating bacilli) are more susceptible to bedaquiline. Additionally, bedaquiline is also effective against nontuberculous mycobacteria, with MICs ranging from 0.06 to 0.5 μg/ml. A potential for the development of resistance to bedaquiline in M. tuberculosis exists. Modification of the atpE target gene, and/or upregulation of the MmpS5-MmpL5 efflux pump (Rv0678 mutations) have been associated with increased bedaquiline MIC values in isolates of M. tuberculosis. Target-based mutations generated in preclinical studies lead to 8- to 133-fold increases in bedaquiline MIC, resulting in MICs ranging from 0.25 to 4 micrograms per mL. Efflux-based mutations have been seen in preclinical and clinical isolates. These lead to 2- to 8-fold increases in bedaquiline MICs, resulting in bedaquiline MICs ranging from 0.25 to 0.5 micrograms per mL. |
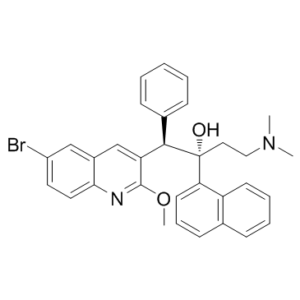
| 分子式 |
C32H31BRN2O2
|
|---|---|
| 分子量 |
555.5
|
| 精确质量 |
554.16
|
| 元素分析 |
C, 69.19; H, 5.62; Br, 14.38; N, 5.04; O, 5.76
|
| CAS号 |
843663-66-1
|
| 相关CAS号 |
Bedaquiline fumarate;845533-86-0;(Rac)-Bedaquiline;654655-80-8;(Rac)-Bedaquiline-d6;2517573-53-2;Bedaquiline impurity 2-d6
|
| PubChem CID |
5388906
|
| 外观&性状 |
White to yellow solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
702.7±60.0 °C at 760 mmHg
|
| 熔点 |
118 °C
|
| 闪点 |
378.8±32.9 °C
|
| 蒸汽压 |
0.0±2.3 mmHg at 25°C
|
| 折射率 |
1.666
|
| LogP |
7.59
|
| tPSA |
36.36
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
37
|
| 分子复杂度/Complexity |
715
|
| 定义原子立体中心数目 |
2
|
| SMILES |
[C@](C1C=CC=C2C=CC=CC=12)(O)(CCN(C)C)[C@H](C1C=CC=CC=1)C1C=C2C=C(C=CC2=NC=1OC)Br
|
| InChi Key |
QUIJNHUBAXPXFS-XLJNKUFUSA-N
|
| InChi Code |
InChI=1S/C32H31BrN2O2/c1-35(2)19-18-32(36,28-15-9-13-22-10-7-8-14-26(22)28)30(23-11-5-4-6-12-23)27-21-24-20-25(33)16-17-29(24)34-31(27)37-3/h4-17,20-21,30,36H,18-19H2,1-3H3/t30-,32-/m1/s1
|
| 化学名 |
(1R,2S)-1-(6-Bromo-2-methoxy-3-quinolyl)-4-dimethylamino-2-(1-naphthyl)-1-phenyl-butan-2-ol
|
| 别名 |
R207910; TMC207; R-207910; TMC-207; R 207910; TMC 207; Bedaquiline; Bedaquiline fumarate; trade name: Sirturo; AIDS-222089; bedaquilina;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : 12.5~33 mg/mL ( 22.50~59.4 mM )
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 0.5 mg/mL (0.90 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 5.0 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 0.5 mg/mL (0.90 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 5.0 mg/mL 澄清 DMSO 储备液加入 900 μL 20% SBE-β-CD 生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 0.5 mg/mL (0.90 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 配方 1 中的溶解度: ≥ 0.5 mg/mL (0.9 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,取100 μL 5 mg/mL DMSO储备液加入400 μL PEG300,混匀(澄清溶液);然后向上述溶液中加入50 μL Tween 80,混匀(澄清溶液);最后,在上述溶液中加入450 μL生理盐水,混匀(澄清溶液)。 *生理盐水的制备:将 0.9 g 氯化钠溶解于 100 mL ddH 2 O 中,得到溶液。 配方 2 中的溶解度: 0.5 mg/mL (0.9 mM)(渗透度未知) in 10% DMSO + 90%(20% SBE-β-CD 生理盐水) (这些助溶剂从左到右依次添加,逐一添加),配制溶液。 例如,如果要制备1 mL工作液,则可以取100 μL 5 mg/mL DMSO储备液并添加到900 μL 20% SBE-β-CD 生理盐水溶液,充分混合(澄清溶液)。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 3 中的溶解度: 0.5 mg/mL (0.9 mM) (饱和度未知) in 10% DMSO + 90% Corn oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,如果要制备1 mL工作液,则可以取100 μL 5 mg/mL DMSO储备液并添加到900 μL 玉米油,混合均匀(澄清溶液)。 配方 5 中的溶解度: 1.67mg/ml (3.01mM) in 5% DMSO + 95% Corn oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.8002 mL | 9.0009 mL | 18.0018 mL | |
| 5 mM | 0.3600 mL | 1.8002 mL | 3.6004 mL | |
| 10 mM | 0.1800 mL | 0.9001 mL | 1.8002 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Distribution of MIC values for rapidly growing mycobacterial strains. The arrows represent the proposed ECOFF value for rapidly growing mycobacteria.Antimicrob Agents Chemother.2017 Apr 24;61(5). pii: e02627-16. |
|---|
Distribution of MIC values for slowly growing mycobacterial strains. The arrows represent the proposed ECOFF value for slowly growing mycobacteria.Antimicrob Agents Chemother.2017 Apr 24;61(5). pii: e02627-16. |