| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
HDAC ( IC50 = 27 nM )
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
体外活性:Belinostat 抑制肿瘤细胞(A2780、HCT116、HT29、WIL、CALU-3、MCF7、PC3 和 HS852)的生长,IC50 为 0.2-0.66 μM。 PD101 在 A2780/cp70 和 2780AD 细胞中显示低活性,这些细胞是 A2780 细胞的顺铂和阿霉素耐药衍生物。 Belinostat 可通过 PARP 裂解和组蛋白 H3/H4 乙酰化诱导细胞凋亡。 Belinostat抑制膀胱癌细胞生长,尤其是5637细胞,表现为G0-G1期积累,S期减少,G2-M期增加。贝利司他对细胞系的生长抑制活性不受多重耐药表型的强烈影响,而多西紫杉醇的活性明显受到影响。 Belinostat 可以增强多西紫杉醇或卡铂对 OVCAR-3 和 A2780 细胞的生长抑制活性。 Belinostat 还显示出卵巢癌细胞系中微管蛋白乙酰化的增强。最近的一项研究表明 Belinostat 通过 TGF-β 信号传导依赖性机制激活蛋白激酶 A,并减少生存素 mRNA。激酶测定:收获亚汇合培养物并在冰冷的 PBS 中洗涤两次,并通过 200 × g 离心 5 分钟沉淀。将细胞沉淀重悬于两体积的裂解缓冲液中[60 mM Tris 缓冲液(pH 7.4),含有 30% 甘油和 450 mM NaCl],并通过三个冷冻(干冰)解冻(30 °C 水浴)循环进行裂解。 1.2×104g离心5分钟去除细胞碎片,上清液保存于-80℃。组蛋白 H4 肽(对应于 20 个 NH2 末端残基的序列 SGRGKGGKGLGKGGAKRHRK)被含有 p300 次黄嘌呤-氨基蝶呤-胸苷结构域的重组蛋白乙酰化,使用 [3H]乙酰辅酶 A 作为乙酸来源。 H4 肽 (100 μg) 与次黄嘌呤-氨基蝶呤-胸苷缓冲液(50 mM Tris HCl pH 8.0、5% 甘油、50 mM KCl 和 0.1 mM EDTA)、1 mM DTT、1 mM 4-(2-氨基乙基) 混合苯磺酰氟、1 × 完全蛋白酶抑制剂、50 μL 纯化的 p300 和 1.85 m [3H]乙酰辅酶A (4.50Ci/mmol),最终体积为 300 μL,并在 30 °C 下孵育 45 分钟。通过与 20 μL 50% Ni-agaroase 珠在 4 °C 下孵育 1 小时并离心来去除 p300 蛋白。将上清液上样至 2 mL Sephadex G15 柱,并收集流出液。轻轻滴加 1 毫升蒸馏水,收集三滴馏分;重复此操作,直到添加 4–5 mL 蒸馏水,并收集~40 个级分。将每个级分的 3 微升稀释在 2 mL 闪烁液中,并在闪烁计数器中计数,以识别含有标记肽的级分。合并这些级分,并测量 1 μL 组合样品,以评估每个肽批次 (3-7×103 cpm/μL) 的放射性。对于活性测定,反应在总体积为 150 μL 的缓冲液 [60 mM Tris (pH 7.4),含有 30% 甘油] 中进行,其中含有 2 μL 细胞提取物和(如果使用)2 μL belinostat。通过添加 2 μL [3H] 标记底物(对应于 20 个 NH2 末端残基的乙酰化组蛋白 H4 肽)开始反应。样品在 37°C 下孵育 45 分钟,并通过添加 HCl 和乙酸(终浓度分别为 0.72 和 0.12 M)终止反应。将释放的[3H]乙酸盐提取到750 μL乙酸乙酯中,并将样品在1.2×104 g下离心5分钟。将上层相 (600 μL) 转移至 3 mL 闪烁液中并计数。细胞测定:将肿瘤细胞系以 8 × 104 个细胞/25 cm2 烧瓶的密度接种在 5 mL 培养基中,并孵育 48 小时。将细胞暴露于 Belinostat(0.016 至 10 μM)24 小时。除去培养基,并向每个烧瓶中添加 1 mL 胰蛋白酶/EDTA。一旦细胞脱离,添加 1 mL 培养基,重新悬浮细胞,并对来自对照未处理烧瓶的细胞进行计数。将细胞稀释并接种到 6 厘米培养皿中(每个烧瓶三个),密度为 0.5-2× 103 个细胞/培养皿,具体取决于细胞系。与对照烧瓶一样,对药物处理烧瓶中的细胞进行稀释和铺板。培养皿在 37°C 下孵育 10-15 天。用 PBS 洗涤细胞,在甲醇中固定,并用结晶紫染色,并对含有 ≥ 50 个细胞的集落进行计数。灵敏度以 IC50 表示,定义为将集落数量减少至对照未处理细胞的 50% 所需的贝利司他浓度。
|
||
| 体内研究 (In Vivo) |
Belinostat 在 10mg/kg 剂量下显示 A2780 和 A2780/cp70 异种移植物中肿瘤生长显着延迟,且对体重没有影响。 Belinostat 还在小鼠膀胱肿瘤中诱导 p21WAF1、HDAC 核心和细胞通讯基因。 Belinostat 单药治疗在 A2780 异种移植物中诱导剂量成比例的抗肿瘤作用,剂量为 100mg/kg 时 TGI 为 47%。 Belinostat (100 mg/kg) 与卡铂 (40 mg/kg) 组合可将肿瘤生长从 18.6 天延迟至 22.5 天。 Belinostat 与硼替佐米联合使用,对硼替佐米耐药 UMSCC-11A 异种移植小鼠产生良好的肿瘤抑制作用和胃肠道毒性
|
||
| 酶活实验 |
收获亚汇合培养物并在冰冷的 PBS 中洗涤两次后,通过以 200 × g 离心 5 分钟来实现亚汇合培养物的颗粒化。将细胞沉淀重悬于两体积裂解缓冲液 [60 mM Tris 缓冲液 (pH 7.4)(含有 30% 甘油和 450 mM NaCl)中后,使用三个冷冻(干冰)解冻(30 °C 水浴)循环进行裂解]。 1.2 × 104 g 离心 5 分钟提取细胞碎片后,将上清液保存在 -80 °C。组蛋白 H4 肽(序列 SGRGKGGKGLGKGGAKRHRK,对应 20 个 NH2 末端残基)被重组蛋白乙酰化,该重组蛋白使用 [3H]乙酰辅酶 A 作为醋酸盐来源,并含有次黄嘌呤-氨基蝶呤-胸苷p300 的结构域。 H4 肽 (100 μg) 与次黄嘌呤-氨基蝶呤-胸苷缓冲液(50 mM Tris HCl pH 8.0、5% 甘油、50 mM KCl 和 0.1 mM EDTA)、1 mM DTT、1 mM 4-(2-氨基乙基) 混合苯磺酰氟、1 × 完全蛋白酶抑制剂、50 μL 纯化的 p300 和 1.85 m [3H]乙酰辅酶A (4.50Ci/mmol),最终体积为 300 μL。然后将混合物在 30°C 下孵育 45 分钟。离心并在 4 °C 下离心 1 小时,从 20 μL 50% Ni-琼脂糖酶珠中提取 p300 蛋白。将上清液上样至 2 mL Sephadex G15 柱后,收集流出液。轻轻加入 1 毫升蒸馏 H2O 并收集三滴馏分后,重复此过程,直到加入 4 至 5 毫升蒸馏 H2O 并收集大约四十个馏分被收集。通过将每个级分的三微升稀释在两毫升闪烁液中并在闪烁计数器中计数结果来鉴定含有标记肽的级分。将这些级分合并,并测量 1 μL 样品以确定每批肽的放射性 (3-7×103 cpm/μL)。活性测定反应在总体积为 150 μL 的缓冲液 [60 mM Tris (pH 7.4),含有 30% 甘油] 中进行,并添加 2 μL 细胞提取物,如果适用,添加 2 μL 贝利司他。添加 20 个 NH2 末端残基的乙酰化组蛋白 H4 肽或 2 μL [3H] 标记底物以启动反应。将样品在 37 °C 下孵育 45 分钟,然后添加乙酸和 HCl(最终浓度分别为 0.72 和 0.12 M)终止反应。将释放的 [3H]乙酸盐萃取到 750 μL 乙酸乙酯中后,将样品以 1.2× 104 g 离心 5 分钟。转移至 3 毫升闪烁液后,对上相 (600 μL) 进行计数。
|
||
| 细胞实验 |
将肿瘤细胞系以 8 × 104 细胞/25 cm2 烧瓶的密度接种到 5 mL 培养基中后,孵育 48 小时。一整天,细胞都接受不同浓度的贝利司他(0.016 至 10 μM)。培养基耗尽后,每个烧瓶接受 1 mL 胰蛋白酶/EDTA。分离后,将细胞重悬于 1 mL 培养基中,并记录未经处理的对照烧瓶中的细胞数量。根据细胞系的不同,将三个细胞稀释并接种到每个烧瓶的 6 cm 培养皿中,密度为 0.5-2× 103 细胞/培养皿。与对照烧瓶类似,药物处理烧瓶的细胞被稀释并铺板。培养皿在 37°C 下孵育十到十五天。将细胞固定在甲醇中,用结晶紫染色,并用 PBS 冲洗后,对含有 50 个或更多细胞的集落进行计数。将集落数量降低至未处理对照细胞的 50% 所需的贝利司他浓度称为 IC50,用于表达敏感性。
|
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Approximately 40% of the belinostat dose is excreted renally, primarily as metabolites and less than 2% of total dose recovered as unchanged parent drug. The volume of distribution is 409 ± 76.7 L. 1240 mL/min Metabolism / Metabolites Primarily metabolized by hepatic UGT1A1. Strong UGT1A1 inhibitors are expected to increase exposure to belinostat. Belinostat also undergoes hepatic metabolism by CYP2A6, CYP2C9, and CYP3A4 enzymes to form belinostat amide and belinostat acid. The enzymes responsible for the formation of methyl belinostat and 3-(anilinosulfonyl)-benzenecarboxylic acid, (3-ASBA) are not known Biological Half-Life Displays a three-compartment pharmacokinetic property with elimination half life of 1.1 hours |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In clinical trials of belinostat in patients with PTCL, the rates of serum enzyme elevations during therapy were usually less than 5%, and were above 5 times the ULN in only 1% to 2% of patients. A single instance of severe acute liver injury leading to death from liver failure was reported in an open label trial of belinostat monotherapy in 120 patients with PTCL. The liver injury arose after 10 cycles of treatment and progressed despite drug discontinuation. Specific details were not provided. In another clinical trial, two cases of cholestatic liver injury were reported but without specific details. Thus, belinostat is considered to be a rare cause of acute liver injury but the timing of onset, associated features, clinical course and outcome have not been well defined. Likelihood score: D (possible cause of clinically apparent liver injury). Protein Binding 92.9% and 95.8% of belinostat is bound to protein. |
||
| 参考文献 | |||
| 其他信息 |
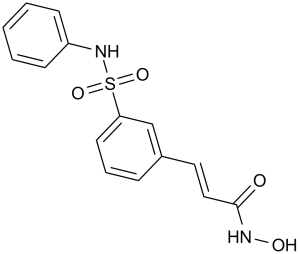
Belinostat is a hydroxamic acid-type histone deacetylase (HDAC) inhibitor with antineoplastic activity. It has a role as an antineoplastic agent and an EC 3.5.1.98 (histone deacetylase) inhibitor. It is a hydroxamic acid, a sulfonamide and an olefinic compound.
Belinostat is a novel agent that inhibits the enzyme histone deacetylase (HDAC) with a sulfonamide-hydroxamide structure. It was developed as an orphan drug to target hematological malignancies and solid tumors by TopoTarget. The safety and efficacy of belinostat is currently being evaluated for use in combination with traditional front-line therapies for the treatment of PTCL. Intravenous administration of the agent is available as Beleodaq as monotherapy and the dosing regimen involves a 21-day cycle. It was US-approved in July 2014 as a therapeutic agent for relapsed or refractory peripheral T-cell lymphoma. Belinostat is a Histone Deacetylase Inhibitor. The mechanism of action of belinostat is as a Histone Deacetylase Inhibitor. Belinostat is an intravenously administered histone deacetylase inhibitor and antineoplastic agent that is approved for use in refractory or relapsed peripheral T cell lymphoma. Belinostat is associated with moderate rate of minor serum enzyme elevations during therapy and has been reported to cause clinically apparent fatal, acute liver injury. Belinostat is a novel hydroxamic acid-type histone deacetylase (HDAC) inhibitor with antineoplastic activity. Belinostat targets HDAC enzymes, thereby inhibiting tumor cell proliferation, inducing apoptosis, promoting cellular differentiation, and inhibiting angiogenesis. This agent may sensitize drug-resistant tumor cells to other antineoplastic agents, possibly through a mechanism involving the down-regulation of thymidylate synthase. Drug Indication Belinostat is indicated for the treatment of patients with relapsed or refractory peripheral T-cell lymphoma (PTCL) with manageable safety profile. It is a potential alternative therapy for patients who did not experience adequate response to first-line drugs for PTCL. It can be used in patients with baseline thrombocytopenia. FDA Label Mechanism of Action Belinostat inhibits the activity of histone deacetylase (HDAC) thus prevents the removal of acetyl groups from the lysine residues of histones and some non-histone proteins. In vitro, belinostat caused the accumulation of acetylated histones and other proteins, increased the expression of tumor-suppressor genes. It ultimately induces cell cycle arrest, inhibition of angiogenesis and/or apoptosis of some transformed cells. Pharmacodynamics Beleodaq is a histone deacetylase (HDAC) inhibitor that exhibits pan-HDAC inhibition and potent growth inhibitory and pro-apoptotic activities in a variety of tumor cells, including PTCL cells, at nanomolar concentrations. None of the trials show any clinically relevant changes caused by Beleodaq on heart rate, PR duration or QRS duration as measures of autonomic state, atrio-ventricular conduction or depolarization; there were no cases of Torsades de Pointes. |
| 分子式 |
C15H14N2O4S
|
|---|---|
| 分子量 |
318.35
|
| 精确质量 |
318.067
|
| 元素分析 |
C, 56.59; H, 4.43; N, 8.80; O, 20.10; S, 10.07.
|
| CAS号 |
414864-00-9
|
| 相关CAS号 |
414864-00-9; 866323-14-0
|
| PubChem CID |
6918638
|
| 外观&性状 |
White solid powder
|
| 密度 |
1.4±0.1 g/cm3
|
| 折射率 |
1.667
|
| LogP |
2.23
|
| tPSA |
103.88
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
22
|
| 分子复杂度/Complexity |
492
|
| 定义原子立体中心数目 |
0
|
| SMILES |
S(C1=C([H])C([H])=C([H])C(/C(/[H])=C(\[H])/C(N([H])O[H])=O)=C1[H])(N([H])C1C([H])=C([H])C([H])=C([H])C=1[H])(=O)=O
|
| InChi Key |
NCNRHFGMJRPRSK-MDZDMXLPSA-N
|
| InChi Code |
InChI=1S/C15H14N2O4S/c18-15(16-19)10-9-12-5-4-8-14(11-12)22(20,21)17-13-6-2-1-3-7-13/h1-11,17,19H,(H,16,18)/b10-9+
|
| 化学名 |
(E)-N-hydroxy-3-[3-(phenylsulfamoyl)phenyl]prop-2-enamide
|
| 别名 |
NSC726630; NSC-726630; PX-105684; PXD 101; PXD101; PXD-101; PX105684; PX 105684; NSC-726630; Trade name: Beleodaq
|
| HS Tariff Code |
2934.99.03.00
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|---|
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.1412 mL | 15.7060 mL | 31.4120 mL | |
| 5 mM | 0.6282 mL | 3.1412 mL | 6.2824 mL | |
| 10 mM | 0.3141 mL | 1.5706 mL | 3.1412 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
A Phase I/II Clinical Trial of PXD101 in Combination with Idarubicin in Patients with AML Not Suitable for Standard Intensive Therapy
CTID: null
Phase: Phase 1, Phase 2 Status: Completed
Date: 2007-06-22
|
|