| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 靶点 |
HDAC6 82 nM (IC50) HDAC 27 nM (IC50, Hela cell)
|
|---|---|
| 体外研究 (In Vitro) |
在肿瘤细胞系中,belinostat (PXD101) 会导致组蛋白 H4 乙酰化增加,且呈浓度依赖性 (0.2–5 μM)。克隆形成研究表明,belinostat 在体外对多种肿瘤细胞系具有细胞毒性,可导致细胞凋亡,IC50 在 0.2–3.4 μM 范围内。许多人类肿瘤细胞系在体外受到贝利司他的抑制,通过克隆实验测量,贝利司他的 IC50 值在 0.2 至 3.4 μM 之间[1]。纯重组 HDAC6 的酶活性(IC50 为 82 nM)可被强组蛋白脱乙酰酶 (HDAC) 抑制剂 belinostat (PXD101) 有效抑制[2]。
|
| 体内研究 (In Vivo) |
连续 7 天,对携带人卵巢和结肠肿瘤异种移植物的裸鼠腹腔注射 Belinostat(10-40 mg/kg/天)。这种治疗显着延迟了动物的生长,但没有显示出任何明显的伤害证据。在卵巢肿瘤异种移植物中发现的对顺铂具有抗性的细胞也表现出生长迟缓。用belinostat (PXD101) 治疗三小时后,小鼠血液和肿瘤中的H4 乙酰化显着升高。小鼠的人类肿瘤异种移植物生长速度较慢,并且没有明显的毒性[1]。当与卡铂疗法联合使用时,belinostat (PXD101) 对人 A2780 卵巢癌皮下异种移植物表现出增强的单药抗肿瘤功效[2]。
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Approximately 40% of the belinostat dose is excreted renally, primarily as metabolites and less than 2% of total dose recovered as unchanged parent drug. The volume of distribution is 409 ± 76.7 L. 1240 mL/min Metabolism / Metabolites Primarily metabolized by hepatic UGT1A1. Strong UGT1A1 inhibitors are expected to increase exposure to belinostat. Belinostat also undergoes hepatic metabolism by CYP2A6, CYP2C9, and CYP3A4 enzymes to form belinostat amide and belinostat acid. The enzymes responsible for the formation of methyl belinostat and 3-(anilinosulfonyl)-benzenecarboxylic acid, (3-ASBA) are not known Biological Half-Life Displays a three-compartment pharmacokinetic property with elimination half life of 1.1 hours |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In clinical trials of belinostat in patients with PTCL, the rates of serum enzyme elevations during therapy were usually less than 5%, and were above 5 times the ULN in only 1% to 2% of patients. A single instance of severe acute liver injury leading to death from liver failure was reported in an open label trial of belinostat monotherapy in 120 patients with PTCL. The liver injury arose after 10 cycles of treatment and progressed despite drug discontinuation. Specific details were not provided. In another clinical trial, two cases of cholestatic liver injury were reported but without specific details. Thus, belinostat is considered to be a rare cause of acute liver injury but the timing of onset, associated features, clinical course and outcome have not been well defined. Likelihood score: D (possible cause of clinically apparent liver injury). Protein Binding 92.9% and 95.8% of belinostat is bound to protein. |
| 参考文献 |
|
| 其他信息 |
Belinostat is a hydroxamic acid-type histone deacetylase (HDAC) inhibitor with antineoplastic activity. It has a role as an antineoplastic agent and an EC 3.5.1.98 (histone deacetylase) inhibitor. It is a hydroxamic acid, a sulfonamide and an olefinic compound.
Belinostat is a novel agent that inhibits the enzyme histone deacetylase (HDAC) with a sulfonamide-hydroxamide structure. It was developed as an orphan drug to target hematological malignancies and solid tumors by TopoTarget. The safety and efficacy of belinostat is currently being evaluated for use in combination with traditional front-line therapies for the treatment of PTCL. Intravenous administration of the agent is available as Beleodaq as monotherapy and the dosing regimen involves a 21-day cycle. It was US-approved in July 2014 as a therapeutic agent for relapsed or refractory peripheral T-cell lymphoma. Belinostat is a Histone Deacetylase Inhibitor. The mechanism of action of belinostat is as a Histone Deacetylase Inhibitor. Belinostat is an intravenously administered histone deacetylase inhibitor and antineoplastic agent that is approved for use in refractory or relapsed peripheral T cell lymphoma. Belinostat is associated with moderate rate of minor serum enzyme elevations during therapy and has been reported to cause clinically apparent fatal, acute liver injury. Belinostat is a novel hydroxamic acid-type histone deacetylase (HDAC) inhibitor with antineoplastic activity. Belinostat targets HDAC enzymes, thereby inhibiting tumor cell proliferation, inducing apoptosis, promoting cellular differentiation, and inhibiting angiogenesis. This agent may sensitize drug-resistant tumor cells to other antineoplastic agents, possibly through a mechanism involving the down-regulation of thymidylate synthase. Drug Indication Belinostat is indicated for the treatment of patients with relapsed or refractory peripheral T-cell lymphoma (PTCL) with manageable safety profile. It is a potential alternative therapy for patients who did not experience adequate response to first-line drugs for PTCL. It can be used in patients with baseline thrombocytopenia. FDA Label Mechanism of Action Belinostat inhibits the activity of histone deacetylase (HDAC) thus prevents the removal of acetyl groups from the lysine residues of histones and some non-histone proteins. In vitro, belinostat caused the accumulation of acetylated histones and other proteins, increased the expression of tumor-suppressor genes. It ultimately induces cell cycle arrest, inhibition of angiogenesis and/or apoptosis of some transformed cells. |
| 分子式 |
C15H14N2O4S
|
|---|---|
| 分子量 |
318.35
|
| 精确质量 |
318.067
|
| CAS号 |
866323-14-0
|
| 相关CAS号 |
866323-14-0; 414864-00-9;
|
| PubChem CID |
6918638
|
| 外观&性状 |
White to off-white solid powder
|
| 熔点 |
160 °C(dec.)
|
| LogP |
4
|
| tPSA |
107.37
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
22
|
| 分子复杂度/Complexity |
492
|
| 定义原子立体中心数目 |
0
|
| SMILES |
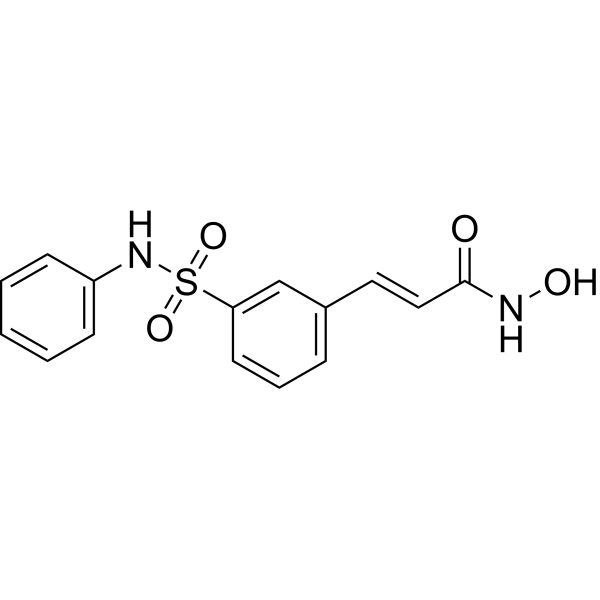
C1=CC=C(C=C1)NS(=O)(=O)C2=CC=CC(=C2)/C=C/C(=O)NO
|
| InChi Key |
NCNRHFGMJRPRSK-MDZDMXLPSA-N
|
| InChi Code |
InChI=1S/C15H14N2O4S/c18-15(16-19)10-9-12-5-4-8-14(11-12)22(20,21)17-13-6-2-1-3-7-13/h1-11,17,19H,(H,16,18)/b10-9+
|
| 化学名 |
(E)-N-hydroxy-3-[3-(phenylsulfamoyl)phenyl]prop-2-enamide
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : 100 mg/mL (314.12 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (7.85 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (6.53 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清的DMSO储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (6.53 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.1412 mL | 15.7060 mL | 31.4120 mL | |
| 5 mM | 0.6282 mL | 3.1412 mL | 6.2824 mL | |
| 10 mM | 0.3141 mL | 1.5706 mL | 3.1412 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。