| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 靶点 |
Caspase-4 (Kd < 0.6 nM); Caspase-1 (Ki = 0.8 nM)
|
|---|---|
| 体外研究 (In Vitro) |
VRT-043198 是 VX-765 的口服吸收前药,对 ICE/caspase-1 和 caspase-4 具有有效抑制作用,Ki 分别为 0.8 nM 和小于 0.6 nM。此外,VRT-043198 还可阻断 PBMC 和全血中 IL-1β 的释放,IC50 值分别为 0.67 μM 和 1.9 μM。 [1]
VRT-43198对半胱氨酸天冬氨酸蛋白酶和其他蛋白酶的选择性。我们在体外评估了VRT-043198对ICE/胱天蛋白酶-1和胱天蛋白酶-4的效力及其对胱天蛋白酶三个亚家族代表和其他蛋白酶的选择性,包括颗粒酶B和胰蛋白酶(丝氨酸蛋白酶)以及组织蛋白酶B(半胱氨酸蛋白酶)。如表1所示,VRT-043198对ICE/胱天蛋白酶-1(Ki=0.8nM)和胱天蛋白酶-4(Ki<0.6nM)表现出强烈的抑制作用,效力至少低100倍。 细胞因子释放[5] 由于有证据表明低温比林参与了IL-1β的产生,我们测量了FCAS患者和对照组PBMC的IL-1β分泌。FCAS患者的PBMC在暴露于LPS 4小时或24小时后分泌的IL-1β水平明显高于未受影响的供体(图1)。在没有LPS处理的情况下释放的IL-1β低于患者和对照组PBMC中的检测水平。暴露于低至0.01 ng/ml的LPS 4小时,对照细胞分泌的IL-1β可以忽略不计,这刺激了FCAS患者PBMC的强劲分泌。在最高浓度LPS(10ng/ml)下释放的IL-1β在FCAS患者的PBMC中高2.1至2.7倍。与ICE/胱天蛋白酶-1参与前IL-1β的加工一致,胱天蛋白酶1抑制剂Belnacasan (VX765)(10μM)显著(>80%)抑制了FCAS和对照细胞释放IL-1β(图1)。 在从单核细胞释放之前,IL-18与IL-1β一样,通过ICE/半胱氨酸天冬氨酸蛋白酶-1进行蛋白水解成熟,因此还测量了细胞培养基中这种细胞因子的水平。IL-18的释放水平比IL-1β低约100倍。与IL-1β一样,FCAS患者的PBMCs中LPS刺激的IL-18释放明显高于对照组,并且在10μM时受到Belnacasan (VX765)的显著抑制(图2)。同样与IL-1β一样,在没有LPS暴露的情况下,没有可检测到IL-18的组成型释放,而在低LPS浓度下,FCAS细胞有显著的IL-18释放,在正常细胞中不产生IL-18释放。 为了确定FCAS PBMCs的异常是单纯的恶性炎症状态还是胱天蛋白酶-1调节的特定缺陷,我们还测量了暴露于LPS 24小时后细胞培养基中的IL-6和IL-1α(图3)。与IL-1β和IL-18形成鲜明对比的是,与对照组相比,FCAS的PBMCs中IL-6的分泌没有增加。尽管只有两名对照组受试者的结果可用,但FCAS中IL-1α的分泌似乎低于对照组(图3)。在最高浓度的LPS(10ng/ml)下,在存在Belnacasan(VX765)(10μM)的情况下,IL-1α的分泌减少了约50%。有趣的是,ICE/胱天蛋白酶-1敲除小鼠的巨噬细胞中IL-1α的分泌减少(18),表明IL-1α分泌部分依赖于ICE/胱天蛋白酶-1或IL-1β水平。 Belnacasan (VX765)的抑制效力[5] 由于FCAS中的突变似乎会影响ICE/胱天蛋白酶-1的活性状态,我们测试了Belnacasan(VX765)抑制ICE/胱天蛋白酶-1介导的IL-1β产生的效力是否会因突变而改变。使用来自三名FCAS和三名对照受试者的PBMCs,在暴露于10ng/ml LPS 24小时后测量,确定了Belnacasan(VX765)抑制IL-1β释放的能力Belnacasan(VX765)抑制FCAS(IC50=0.99±0.29μM)和对照(IC50=1.10±0.61μM)受试者外周血单个核细胞中IL-1β的释放,其效力相似(图5)。 |
| 体内研究 (In Vivo) |
在胶原诱导的关节炎小鼠模型中,VX-765 (200 mg/kg) 可抑制 LPS 诱导的 IL-1β 产生约 60%,导致炎症评分呈剂量依赖性且具有统计学显着性降低,并提供有效的关节保护。 [1]在不显着影响后放电长度的情况下,VX-765 通过阻止前脑星形胶质细胞中 IL-1β 的生长来阻断大鼠体内点燃癫痫发生。 [2]在急性癫痫发作的小鼠模型中,VX-765(50 mg/kg-200 mg/kg)通过延迟首次癫痫发作的时间并平均减少癫痫发作次数及其总持续时间来产生抗惊厥作用分别为 50% 和 64%。[3]第三次注射后,VX-765 通过特异性阻断 IL-1 生物合成,显着降低遗传性失神性癫痫 (GAERS) 成年大鼠的棘波放电 (SWD) 累积持续时间和数量,从而导致平均 55%。[4]
Belnacasan(VX765)在小鼠口服给药时可有效转化为VRT-043198,并抑制脂多糖诱导的细胞因子分泌。此外,VX-765降低了类风湿性关节炎和皮肤炎症模型中的疾病严重程度和炎症介质的表达。这些数据表明,VX-765是一种新型的细胞因子抑制剂,可用于治疗炎症性疾病。[1] 胶质细胞中IL-1β的增加是实验模型和人类药物难治性癫痫中致痫组织的典型特征。我们在这里表明,白细胞介素转换酶(ICE)的选择性抑制可以通过阻止前脑星形胶质细胞中IL-1β的增加来阻断大鼠的点燃发展,而不会干扰胶质细胞的激活。Belnacasan (VX765)对平均后放电持续时间没有显著影响。在点燃完成和药物洗脱后24小时内,治疗组大鼠无法诱发点燃性癫痫发作。Belnacasan (VX765)对完全点燃的大鼠的癫痫发作或放电后持续时间没有影响。这些数据表明,ICE抑制介导了抗癫痫作用,并表明可以设想特定的抗IL-1β药理学策略来干扰癫痫发生机制。[2] 在这项研究中,在一种慢性癫痫小鼠模型中检测了Belnacasan (VX765)(一种选择性ICE/caspase-1抑制剂)的抗惊厥活性,该模型具有对一些常见抗惊厥药物无效的自发复发性癫痫活动。此外,在一种小鼠急性癫痫发作模型中研究了这种药物的作用,该模型先前已被证明涉及ICE/半胱氨酸天冬氨酸蛋白酶-1的激活。对暴露于急性癫痫发作或癫痫持续状态后出现慢性癫痫活动的小鼠进行脑电图活动的定量分析,以评估全身给药Belnacasan (VX765)的抗惊厥作用。在癫痫小鼠药理学实验结束时,对脑组织进行组织学和免疫组织化学分析,以评估神经病理学、胶质细胞活化和IL-1β表达以及治疗效果。重复全身给药VX-765以剂量依赖的方式显著降低了小鼠的慢性癫痫活动(12.5-200mg/kg)。在剂量≥50mg/kg时观察到这种作用,并且停药后可以逆转。最大药物效应与抑制活化星形胶质细胞中IL-1β的合成有关。VX-765的相同剂量方案也减少了小鼠的急性癫痫发作,并延迟了发作时间。这些结果支持了一种新的抗惊厥药物干预靶系统,以控制对一些常见抗惊厥药物没有反应的癫痫活动[3]。 |
| 酶活实验 |
监测用对硝基苯胺或氨甲基香豆素 (AMC) 标记的合适底物的水解速率以确定酶是否被抑制:粒酶 B、Ac-IEPD-AMC; caspase-3、-7、-8 和 -9; caspase-4,Ac-WEHD-AMC; caspase-6,Ac-VEID-AMC;和 ICE/caspase-1,suc-YVAD-p-硝基苯胺。反应缓冲液含有 10 mM Tris、pH 7.5、0.1% (w/v) CHAPS、1 mM 二硫苏糖醇和 5% (v/v) 二甲亚砜,与酶和底物在 37 ° 下孵育 10 分钟C。为了提高 caspase-3、-6 和 -9 以及颗粒酶 B 的稳定性,将甘油以 8% (v/v) 的浓度添加到缓冲液中。使用荧光计测量底物水解速率。
|
| 细胞实验 |
通过测定VRT-043198对体外单核细胞释放细胞因子的影响,以及在几种动物模型中口服VX-765对体内细胞因子释放的影响,评估VX-765的治疗潜力。在受细菌产物刺激的健康人外周血单个核细胞和全血培养中,VRT-043198抑制白细胞介素(IL)-1 β和IL-18的释放,但对其他几种细胞因子的释放影响不大,包括IL-1 α、肿瘤坏死因子α、IL-6和IL-8。相比之下,VRT-043198在细胞凋亡模型中几乎没有或没有明显的活性,并且不影响活化的原代T细胞或T细胞系的增殖。小鼠口服VX-765可有效转化为VRT-043198,抑制脂多糖诱导的细胞因子分泌。此外,VX-765降低了类风湿性关节炎和皮肤炎症模型的疾病严重程度和炎症介质的表达。这些数据表明,VX-765是一种新的细胞因子抑制剂,可用于治疗炎症性疾病。[1]
在接触 LPS 之前,用 VX-765 将 PBMC 预处理 30 分钟。 分泌细胞因子测定[5] 共2×105个细胞/孔(100μl细胞悬浮液)在平底96孔板上分布三份。将50μl的Belnacasan(VX765)(在含有0.2%DMSO的RPMI 1640完全培养基中为40μM)或载体对照加入适当的孔中。在37°C下孵育30分钟后,加入50μl在RPMI 1640完全培养基中稀释的LPS,终浓度为0.001至10ng/ml。将细胞放回37°C的培养箱中。在添加LPS后4小时,从孔中取出75μl上清液,以1500 rpm离心5分钟进行清除,并在4°C下储存,直至进行检测。将细胞放回37°C的培养箱中,直到添加LPS后24小时,此时去除100μl上清液,离心清除,并储存在4°C下。根据制造商的说明,使用ELISA试剂盒检测上清液中的IL-1β、IL-6、IL-18和IL-1α。 免疫印迹[5] 共有106个细胞/孔(500μl细胞悬浮液)分布在24孔板中。如上所述,用Belnacasan(VX765)和LPS处理细胞,调节终体积为1ml。加入LPS 4小时后,将所有培养基转移到1.5ml试管中,在4°C下以1000×g离心5分钟,使悬浮细胞沉淀,并去除上清液。为了裂解附着的细胞,向每个孔中加入100μl 1×NuPage样品缓冲液和2-ME,并将平板放置在轨道振荡器上。将孔中的100μl样品缓冲液转移到相应的颗粒中,煮沸15分钟,然后在-20°C下冷冻。在装载到4-12%的Bis-Tris NuPage凝胶上之前,再次煮沸样品,并用NuPage MES运行缓冲液运行。凝胶被转移到硝化纤维过滤器中。过滤器用TBST(0.05%吐温20)+5%干乳吸干。对于IL-1β免疫印迹,印迹在4°C下与1:2000小鼠抗IL-1β初级抗体孵育过夜,然后与1:10000 HRP山羊抗小鼠次级抗体孵育1小时,并用ECL显影。 |
| 动物实验 |
Mice: Belnacasan is injected intravenously as single doses (10, 21, 43, and 84 mg/kg) in a vehicle (25% Cremophor EL in water). Through the retroorbital sinus, blood samples (roughly 0.25-0.3 mL) are taken before the dose is administered as well as 0.167, 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, and 8 h later. These samples are processed for plasma. The concentration of Belnacasan and VRT-043198 is measured in plasma samples using a high-performance liquid chromatography/mass spectrometry methodology. Using WinNonlin Pro, version 4.0.1, noncompartmental analysis is performed.
Rats: Male Sprague-Dawley rats weighing 250–280 g are employed. Belnacasan (25, 50, or 200 mg/kg) is dissolved in 20% Cremophor and injected intraperitoneally (i.p.) into rats once daily for three straight days. Rats are given Belnacasan on the fourth day, 45 minutes and 10 minutes before intrahippocampal injections of kainic acid. Prior to the injection of kainic acid, respective controls receive a similar vehicle injection. Kindling development [2] Belnacasan (VX765) (200 mg/kg) was dissolved in 20% cremophor and injected intraperitoneally (i.p.) in rats once a day for 3 consecutive days (n = 12); control rats (n = 12) received the corresponding vehicle. On the 4th day, rats received Belnacasan (VX765) or vehicle, 45 min before the beginning of the electrical stimulation. We adopted this treatment protocol since it provided significant protection from seizures induced by intrahippocamapl injection of kainic acid in rats (Ravizza et al., 2006b), and this effect was associated with inhibition of pro-IL-1β processing and of the consequent production of the biologically active form of IL-1β in the hippocampus (Ravizza et al., 2006b). During the stimulation protocol VX-765, or its vehicle, was injected 3 times every 90 min since previous experiments showed that the drug effect on kainic acid--induced seizures was maintained for 90 min slowly decreasing thereafter (Ravizza et al., 2006b). In preliminary experiments, VX-765 was also administered at 50 mg/kg (n = 5) but this dose did not affect kindling parameters (not shown). Fully kindled rats [2] After the re-test session (24 h after kindling completion), rats (n = 7) were treated for 3 consecutive days with Belnacasan (VX765), as described above; on the 4th day, Belnacasan (VX765) was administered once, and 45 min later rats received 5 electrical stimulations to evoke fully kindled seizures. The same rats, after 3 days of drug washout, received vehicle using the same treatment and stimulation protocol adopted with VX-765. |
| 药代性质 (ADME/PK) |
Based upon the data provided in this panel, it was clear that these agents represent important new tools for caspase 1 inhibition. However, the contributing functional groups for these agents (i.e. ethyl acetals, aldehydes, nitriles and esters) are all subject to hydrolysis in various conditions. It was paramount to fully understand their stability profile to appreciate their utility as molecular probes or even clinically used agents. Therefore, we examined 1/Belnacasan (VX765) , 2b, 3, 4 and 16 within an aqueous degradation study at neutral (pH 7), acidic (pH 2), and basic (pH 8) conditions. The study was conducted by monitoring the degradation of each agent by LCMS analysis at various time points over 96 hours (Figure 3). The prodrug 1Belnacasan (VX765) / showed moderate degradation in water with over 50% of the compound decomposed after 48 hours. This degradation was amplified in both basic and acidic conditions. Conversely, the active agent 2b was very stable in both neutral and acidic conditions and its degradation at pH 8 was moderate. The potent 4 was exceedingly stable in basic conditions and its stability in neutral and acidic conditions was moderate to good (degradation of 50% in both conditions after 72 hours). The ethyl ester 3 was exceptionally stable in neutral and acidic conditions (no degradation noted), however, it was fully degraded in basic conditions after 22 hours (presumably due to saponification of the ester). Finally, the tetrazole 16 was found to be resistant to degradation in all conditions. Interestingly, this data suggests that 1/Belnacasan (VX765) may have a short half-life as an oral agent due to its instability in acidic conditions such as those found in the gastric environment (40% degradation after 3.5 hours at pH 2). In contrast, this data highly suggests that 3 and 16 will be suitable reagents for all manner of examinations (cell based and in vivo studies) and even the highly active 4 will persist beyond 24 hours.[https://pubmed.ncbi.nlm.nih.gov/20229566/]
|
| 参考文献 | |
| 其他信息 |
Belnacasan is a dipeptide.
VX-765 is the orally available prodrug of a potent and selective competitive inhibitor of ICE/caspase-1 (VRT-043198). VX-765 is currently under clinical development for the treatment of inflammatory and autoimmune conditions, as it blocks the hypersensitive response to an inflammatory stimulus. Drug Indication Investigated for use/treatment in inflammatory disorders (unspecified) and psoriasis and psoriatic disorders. Mechanism of Action VX-765 is a potent and selective inhibitor of ICE/caspase-1 sub-family caspases. In preclinical trials, VX-765 was efficiently converted to VRT-043198 when administered orally to mice and inhibited LPS-induced cytokine secretion. The result was a blocking of IL-1beta and IL-18 secretion, with out much effect on the release of several other cytokines, including IL-1{alpha}, tumor necrosis factor-{alpha}, IL-6 and IL-8. There was also no demonstrable activity in cellular models of apoptosis and it did not affect the proliferation of activated primary T-cells or T-cell lines. Pharmacodynamics VX-765 is an orally-absorbed pro-drug of VRT-043198, a potent and selective inhibitor of ICE/caspase-1 sub-family caspases. It has been shown to reduce disease severity and the expression of inflammatory mediators in models of rheumatoid arthritis and skin inflammation, suggesting that it may be useful for treatment of inflammatory diseases. (S)-1-((S)-2-{[1-(4-amino-3-chloro-phenyl)-methanoyl]-amino}-3,3-dimethyl-butanoyl)-pyrrolidine-2-carboxylic acid ((2R,3S)-2-ethoxy-5-oxo-tetrahydro-furan-3-yl)-amide (VX-765) is an orally absorbed prodrug of (S)-3-({1-[(S)-1-((S)-2-{[1-(4-amino-3-chlorophenyl)-methanoyl]-amino}-3,3-dimethyl-butanoyl)-pyrrolidin-2yl]-methanoyl}-amino)-4-oxo-butyric acid (VRT-043198), a potent and selective inhibitor of interleukin-converting enzyme/caspase-1 subfamily caspases. VRT-043198 exhibits 100- to 10,000-fold selectivity against other caspase-3 and -6 to -9. The therapeutic potential of VX-765 was assessed by determining the effects of VRT-043198 on cytokine release by monocytes in vitro and of orally administered VX-765 in several animal models in vivo. In cultures of peripheral blood mononuclear cells and whole blood from healthy subjects stimulated with bacterial products, VRT-043198 inhibited the release of interleukin (IL)-1beta and IL-18, but it had little effect on the release of several other cytokines, including IL-1alpha, tumor necrosis factor-alpha, IL-6 and IL-8. In contrast, VRT-043198 had little or no demonstrable activity in cellular models of apoptosis, and it did not affect the proliferation of activated primary T cells or T-cell lines. VX-765 was efficiently converted to VRT-043198 when administered orally to mice, and it inhibited lipopolysaccharide-induced cytokine secretion. In addition, VX-765 reduced disease severity and the expression of inflammatory mediators in models of rheumatoid arthritis and skin inflammation. These data suggest that VX-765 is a novel cytokine inhibitor useful for treatment of inflammatory diseases. [1] Interleukin (IL)-1β plays a crucial role in the mechanisms of limbic seizures in rodent models of temporal lobe epilepsy. We addressed whether activation of the IL-1β signaling occurs in rats with genetic absence epilepsy (GAERS) during the development of spike-and-wave discharges (SWDs). Moreover, we studied whether inhibition of IL-1β biosynthesis in GAERS could affect SWD activity. IL-1β expression and glia activation were studied by immunocytochemistry in the forebrain of GAERS at postnatal days (PN)14, PN20, and PN90 and in age-matched non-epileptic control Wistar rats. In PN14 GAERS, when no SWDs have developed yet, IL-1β immunostaining was undetectable, and astrocytes and microglia showed a resting phenotype similar to control Wistar rats. In 3 out of 9 PN20 GAERS, IL-1β was observed in activated astrocytes of the somatosensory cortex; the cytokine expression was associated with the occurrence of immature-type of SWDs. In all adult PN90 GAERS, when mature SWDs are established, IL-1β was observed in reactive astrocytes of the somatosensory cortex but not in adjacent cortical areas or in extra-cortical regions. An age-dependent c-fos activation was found in the somatosensory cortex of GAERS with maximal levels reached in PN90 rats; c-fos was also induced in some thalamic nuclei in PN20 and PN90 GAERS. Inhibition of IL-1β biosynthesis in PN90 GAERS by 4-day systemic administration of a specific ICE/Caspase-1 blocker, significantly reduced both SWD number and duration. These results show that IL-1β is induced in reactive astrocytes of the somatosensory cortex of GAERS at the onset of SWDs. IL-1β has pro-ictogenic properties in this model, and thus it may play a contributing role in the mechanisms underlying the occurrence of absence seizures. [4] Familial cold autoinflammatory syndrome (FCAS) and the related autoinflammatory disorders, Muckle-Wells syndrome and neonatal onset multisystem inflammatory disease, are characterized by mutations in the CIAS1 gene that encodes cryopyrin, an adaptor protein involved in activation of IL-converting enzyme/caspase-1. Mutations in cryopyrin are hypothesized to result in abnormal secretion of caspase-1-dependent proinflammatory cytokines, IL-1beta and IL-18. In this study, we examined cytokine secretion in PBMCs from FCAS patients and found a marked hyperresponsiveness of both IL-1beta and IL-18 secretion to LPS stimulation, but no evidence of increased basal secretion of these cytokines, or alterations in basal or stimulated pro-IL-1beta levels. VX-765, an orally active IL-converting enzyme/caspase-1 inhibitor, blocked IL-1beta secretion with equal potency in LPS-stimulated cells from FCAS and control subjects. These results further link mutations in cryopyrin with abnormal caspase-1 activation, and support the clinical testing of caspase-1 inhibitors such as VX-765 in autoinflammatory disorders. [5] |
| 分子式 |
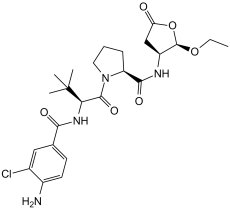
C24H33CLN4O6
|
|
|---|---|---|
| 分子量 |
508.99
|
|
| 精确质量 |
508.208
|
|
| 元素分析 |
C, 56.63; H, 6.53; Cl, 6.97; N, 11.01; O, 18.86
|
|
| CAS号 |
273404-37-8
|
|
| 相关CAS号 |
|
|
| PubChem CID |
11398092
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.3±0.1 g/cm3
|
|
| 沸点 |
779.0±60.0 °C at 760 mmHg
|
|
| 闪点 |
424.9±32.9 °C
|
|
| 蒸汽压 |
0.0±2.7 mmHg at 25°C
|
|
| 折射率 |
1.589
|
|
| LogP |
0.83
|
|
| tPSA |
147.04
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
7
|
|
| 可旋转键数目(RBC) |
8
|
|
| 重原子数目 |
35
|
|
| 分子复杂度/Complexity |
818
|
|
| 定义原子立体中心数目 |
4
|
|
| SMILES |
ClC1=C(C([H])=C([H])C(=C1[H])C(N([H])[C@]([H])(C(N1C([H])([H])C([H])([H])C([H])([H])[C@@]1([H])C(N([H])[C@@]1([H])C([H])([H])C(=O)O[C@@]1([H])OC([H])([H])C([H])([H])[H])=O)=O)C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H])=O)N([H])[H]
|
|
| InChi Key |
SJDDOCKBXFJEJB-MOKWFATOSA-N
|
|
| InChi Code |
InChI=1S/C24H33ClN4O6/c1-5-34-23-16(12-18(30)35-23)27-21(32)17-7-6-10-29(17)22(33)19(24(2,3)4)28-20(31)13-8-9-15(26)14(25)11-13/h8-9,11,16-17,19,23H,5-7,10,12,26H2,1-4H3,(H,27,32)(H,28,31)/t16-,17-,19+,23+/m0/s1
|
|
| 化学名 |
(2S)-1-[(2S)-2-[(4-amino-3-chlorobenzoyl)amino]-3,3-dimethylbutanoyl]-N-[(2R,3S)-2-ethoxy-5-oxooxolan-3-yl]pyrrolidine-2-carboxamide
|
|
| 别名 |
Belnacasan; VX 765; VX765; Belnacasan (VX-765); Belnacasan (VX765); Belnacasan [USAN]; (S)-1-((S)-2-(4-amino-3-chlorobenzamido)-3,3-dimethylbutanoyl)-N-((2R,3S)-2-ethoxy-5-oxotetrahydrofuran-3-yl)pyrrolidine-2-carboxamide; VX-765
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~100 mg/mL (~196.5 mM)
Ethanol: ~100 mg/mL (~196.5 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 3.33 mg/mL (6.54 mM) in 15% Cremophor EL + 85% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。
*生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (4.91 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (4.91 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: ≥ 2.5 mg/mL (4.91 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL 澄清 DMSO 储备液加入900 μL 玉米油中,混合均匀。 配方 5 中的溶解度: 2% DMSO+30% PEG 300+ddH2O: 5mg/mL 配方 6 中的溶解度: 5 mg/mL (9.82 mM) in 50% PEG300 + 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9647 mL | 9.8234 mL | 19.6468 mL | |
| 5 mM | 0.3929 mL | 1.9647 mL | 3.9294 mL | |
| 10 mM | 0.1965 mL | 0.9823 mL | 1.9647 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05164120 | Completed | Drug: Placebo Drug: Belnacasan |
COVID-19 | MedStar Health | December 14, 2021 | Phase 2 |
|
|---|
|
|
|
|---|