| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg | |||
| Other Sizes |
| 靶点 |
Histamine H1 receptor
|
|---|---|
| 体外研究 (In Vitro) |
Bepotastine(10、100、1000 µM;预孵育 120 分钟)可减少 A23187 治疗诱导的组胺释放,在 1000 µM 时达到统计学上显着的减少水平[1]。 Bepotastine(50 µM;1 小时)可抑制 NHEK 中 NGF mRNA 的表达[2]。细胞活力测定[1] 细胞系:RPMC 浓度:10、100、1000 µM 孵育时间:120 分钟(预孵育) 结果:组胺释放减少。 Western Blot 分析[2] 细胞系:NHEK 浓度:50 µM(预孵育) 孵育时间:1 小时 结果:抑制 NHEK 中 NGF mRNA 的表达。
|
| 体内研究 (In Vivo) |
贝托斯汀(10 g/L;滴眼剂;一只眼睛 3 次,间隔 20 分钟)显示出对 PAF 诱导的结膜嗜酸性粒细胞浸润的显着抑制作用[1]。贝托斯汀(3 mg/kg;口服;一次)抑制抓挠行为的频率为 59.0,持续时间为 14.57 秒,与对照组相比几乎处于相同水平[3]。 Bepotastine(10 mg/kg;口服;一次)在出现皮疹的 NC/Nga 小鼠中显着抑制血清 LTB 4 水平,1 小时时为 711.3 pg/mL,2 小时时为 858.8 pg/mL[3]。动物模型:豚鼠(6周龄)[1]。剂量:10 µL 为 10 g/L (1.0% (w/v))。给药方法:滴眼剂; 3次,间隔20分钟(一只眼睛)。结果:抑制PAF诱导的结膜嗜酸性粒细胞浸润。动物模型:雄性BALB/c小鼠(12周龄); NC/Nga 小鼠[3]。剂量:3、10 mg/kg 给药方法:口服;一次(诱导雄性 BALB/c 小鼠抓挠行为前 1 小时)。结果:显着抑制雄性 BALB/c 小鼠组胺介导的抓挠行为。显着抑制出现皮疹的 NC/Nga 小鼠的血清 LTB 4 水平。
|
| 细胞实验 |
细胞系:RPMC
浓度:10、100、1000 µM 孵育时间:120 分钟(预孵育) 结果:组胺释放减少 |
| 动物实验 |
Guinea pigs (6-week-old)
10 g/L (1.0% (w/v)) for 10 µL Eye drop; 3 times at intervals of 20 min (in one eye). |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Tmax, after single dose, opthalmic = 1.2 hours; Cmax, 1.5%, opthalmic dose = 7.3 ±1.9 ng/mL; After 24 hours post-installation, levels of bepotastine are below quantifiable limit of 2 ng/mL. Minimal systemic absorption with opthalmic dosage form. When a oral dose of 2.5 - 40 mg bepotastine is given, 75%-90% of the dose was excreted unchanged in the urine by 24 hours. Metabolism / Metabolites Minimal metabolism via CYP enzymes Biological Half-Life Elimination half life = 2.5 hours |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation There are no reports of infants breastfed during maternal therapy with bepotastine. Because absorption from the eye is limited, bepotastine would not be expected to cause any adverse effects in breastfed infants. To substantially diminish the amount of drug that reaches the breastmilk after using eye drops, place pressure over the tear duct by the corner of the eye for 1 minute or more, then remove the excess solution with an absorbent tissue. ◉ Effects in Breastfed Infants Relevant published information on bepotastine was not found as of the revision date. In one telephone follow-up study, mothers reported irritability and colicky symptoms in 10% of infants exposed to various antihistamines and drowsiness was reported in 1.6% of infants. None of the reactions required medical attention and none of the patients were using bepotastine. ◉ Effects on Lactation and Breastmilk Antihistamines in relatively high doses given by injection can decrease basal serum prolactin in nonlactating women and in early postpartum women. However, suckling-induced prolactin secretion is not affected by antihistamine pretreatment of postpartum mothers. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. Low ophthalmic doses of bepotastine are unlikely to have the same effect on serum prolactin. Protein Binding 55.4% mean plasma protein binding with 10 mg oral dose. Extent of protein binding is independent of plasma drug concentration. |
| 参考文献 |
|
| 其他信息 |
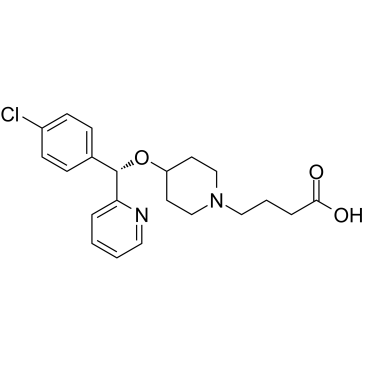
Bepotastine is an ether that is (S)-(4-chlorophenyl)(pyridin-2-yl)methanol in which the hydroxyl hydrogen is substituted by a 1-(3-carboxypropyl)piperidin-4-yl group. A topical, selective and non-sedating histamine (H1) receptor antagonist used (as its benzenesulfonate salt) for treatment of itching associated with allergic conjunctivitis. It has a role as a H1-receptor antagonist and an anti-allergic agent. It is a member of pyridines, a monocarboxylic acid, a member of piperidines, an ether and a member of monochlorobenzenes. It is a conjugate base of a bepotastine(1+).
Bepotastine is a non-sedating, selective antagonist of the histamine 1 (H1) receptor. Bepotastine was approved in Japan for use in the treatment of allergic rhinitis and uriticaria/puritus in July 2000 and January 2002, respectively, and is marketed by Tanabe Seiyaku Co., Ltd. under the brand name Talion. It is available in oral and opthalmic dosage forms in Japan. The opthalmic solution is FDA approved since Sept 8, 2009 and is under the brand name Bepreve. Bepotastine is a Histamine-1 Receptor Antagonist. Drug Indication For the symptomatic treatment of itchy eyes (caused by IgE-induced mast cell degranulation) due to allergic conjunctivitis. FDA Label Mechanism of Action Because of a type 1 hypersensitivity reaction cascade that is triggered by antigen exposure, allergic conjunctivitis occurs. Allergen exposure is followed by conjunctival mast cell degranulation and histamine released as a result of the formation of complementary IgE cross-links on the conjunctiva. Due to the release of histamine, symptoms such as itching can be observed. Bepotastine works to relieve itchy eyes by three primary mechanisms of action. It is a non-sedating, selective antagonist of the histamine 1 (H1) receptor, a mast cell stabilizer, and it suppresses the migration of eosinophils into inflamed tissues to prevent tissue damage and worsening of allergic inflammation of the conjunctiva. Pharmacodynamics Bepotastine is a non-sedating, selective antagonist of the histamine 1 (H1) receptor. It belongs to the second-generation piperidine chemical class. It is a mast cell stabilizer and suppresses the migration of eosinophils into inflamed tissues. Furthermore, bepotastine does not interact with serotonin, muscarinic, benzodiazepine, and beta-adrenergic receptor that would otherwise result in adverse reactions such as dry mouth or sonmolence. Onset of action = 0.25 hours; Duration of action = 12-24 hours; |
| 分子式 |
C21H25CLN2O3
|
|---|---|
| 分子量 |
388.8878
|
| 精确质量 |
388.155
|
| 元素分析 |
C, 64.86; H, 6.48; Cl, 9.12; N, 7.20; O, 12.34
|
| CAS号 |
125602-71-3
|
| 相关CAS号 |
Bepotastine besilate; 190786-44-8; Bepotastine tosylate; 1160415-45-1; 125602-71-3
|
| PubChem CID |
164522
|
| 外观&性状 |
Light brown to brown liquid
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
546.8±50.0 °C at 760 mmHg
|
| 熔点 |
56-58 °C
|
| 闪点 |
284.5±30.1 °C
|
| 蒸汽压 |
0.0±1.5 mmHg at 25°C
|
| 折射率 |
1.605
|
| LogP |
3.67
|
| tPSA |
62.66
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
27
|
| 分子复杂度/Complexity |
449
|
| 定义原子立体中心数目 |
1
|
| SMILES |
C1=CC=NC(=C1)[C@H](C2=CC=C(C=C2)Cl)OC3CCN(CCCC(=O)O)CC3
|
| InChi Key |
YWGDOWXRIALTES-NRFANRHFSA-N
|
| InChi Code |
InChI=1S/C21H25ClN2O3/c22-17-8-6-16(7-9-17)21(19-4-1-2-12-23-19)27-18-10-14-24(15-11-18)13-3-5-20(25)26/h1-2,4,6-9,12,18,21H,3,5,10-11,13-15H2,(H,25,26)/t21-/m0/s1
|
| 化学名 |
4-[4-[(S)-(4-chlorophenyl)-pyridin-2-ylmethoxy]piperidin-1-yl]butanoic acid
|
| 别名 |
TAU-284; TAU 284; TAU284; Bepotastine band name: Talion; Bepreve
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: 78~100 mg/mL (200.6~257.1 mM)
Water: ~78 mg/mL Ethanol: ~78 mg/mL |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.43 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (6.43 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (6.43 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5714 mL | 12.8571 mL | 25.7142 mL | |
| 5 mM | 0.5143 mL | 2.5714 mL | 5.1428 mL | |
| 10 mM | 0.2571 mL | 1.2857 mL | 2.5714 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT01900054 | Completed | Drug: Bepotastine besilate | Perennial Allergic Rhinitis | Mitsubishi Tanabe Pharma Corporation |
June 2013 | Phase 3 |
| NCT01861522 | Completed | Drug: Bepotastine besilate Drug: Placebo |
Perennial Allergic Rhinitis | Mitsubishi Tanabe Pharma Corporation |
April 2013 | Phase 3 |
| NCT01840605 | Completed | Drug: Bepotastine besilate Drug: ketotifen fumarate |
Dermatitis Atopic |
Mitsubishi Tanabe Pharma Corporation |
March 2013 | Phase 3 |
| NCT01425632 | Completed | Drug: TAU-284 Drug: Placebo |
Perennial Allergic Rhinitis | Mitsubishi Tanabe Pharma Corporation |
August 2011 | Phase 3 |
| NCT00586625 | Completed | Drug: Bepreve Drug: Placebo |
Allergic Conjunctivitis | Bausch & Lomb Incorporated | October 2007 | Phase 3 |