| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
p38α (IC50 = 38 nM); p38β (IC50 = 65 nM); p38δ (IC50 = 520 nM); p38γ (IC50 = 200 nM); B-Raf (IC50 = 83.4 nM); Abl (IC50 = 14600 nM); p38 MAP kinase (Kd = 0.1 nM)
|
|---|---|
| 体外研究 (In Vitro) |
BIRB 796 对 ERK-1、SYK、IKK2β、ZAP-70、EGF 受体激酶、HER2、蛋白激酶 A (PKA)、PKC、PKC-α、PKC-β(I 和 II)和 PKC-γ 无显着抑制作用。通过在吗啉氧和 p38α 的 ATP 结合结构域之间形成氢键,BIRB 796 显着提高了结合亲和力。人类 p38 MAP 激酶抑制剂 BIRB 796 是目前最有效且解离缓慢的抑制剂之一。 [1] BIRB 796 有效抑制 c-Raf-1 和 Jnk2α2,IC50 分别为 1.4 和 0.1 nM。 [2] 此外,BIRB796 抑制 SAPK3/p38γ 活性和激活的浓度高于对 p38α 的抑制浓度。支架蛋白 SAP97 是 SAPK3/p38γ 的生理底物,在应激条件下会被磷酸化,但 BIRB796 可以防止这种情况发生。在 HEK293 细胞中,BIRB796 抑制 JNK1/2 激活和活性,但对 Hela 细胞中 ERK1/ERK2 激活或活性没有影响。此外,BIRB796 与 p38 MAPK 或 JNK1/2 的结合不是促进去磷酸化,而是通过上游激酶 MKK6 或 MKK4 抑制它们的磷酸化。 [3] 通过阻断基线和硼替佐米诱导的 p38 MAPK 和 Hsp27 磷酸化上调,BIRB 796 增加细胞毒性和 caspase 激活。受 TNF-α 和 TGF-β1 刺激的 BMSC 会分泌 IL-6 和 VEGF,这些物质会被 BIRB 796 下调。 [4] BIRB-796 的吡唑支架在较低选择性位点放置一个亲脂性叔丁基和一个甲苯环在上部选择性位点。此外,BIRB-796 抑制 B-Raf 和 Abl,IC50 值分别为 83 nM 和 14.6 μM。 [5]
|
| 体内研究 (In Vivo) |
BIRB 796 (30 mg/kg) 可抑制经 LPS 刺激的小鼠 84% 的 TNF-α,并且在胶原诱导的关节炎小鼠模型中显示出有效性。 [1] BIRB 796 BIRB 796 即使在小鼠口服给药后也表现出良好的药代动力学性能。 [2]
|
| 酶活实验 |
THP-1 细胞预孵育 30 分钟。含或不含 BIRB 796 均可。将 LPS 添加至细胞混合物中,终浓度为 1 μg/mL,并进行上述孵育过夜(18-24 小时)。使用市售的 ELISA 来检查上清液中的人 TNF-α。 EC50 值是通过组合数据并使用三参数逻辑模型执行非线性回归分析来计算的。在每个实验中,都会检查 BIRB 796,EC50 的 95% 置信区间范围为 16 至 22 nM。
迄今为止开发的大多数激酶抑制剂都是靶向ATP结合位点的竞争性抑制剂;然而,格列卫(甲磺酸伊马替尼,STI571,PDB:1IEP)、耐克伐尔(甲苯磺酸索拉非尼,BAY 43-9006,PDB:1UWJ)和BIRB 796 (Doramapimod) (PDB:1KV2)的最新晶体结构揭示了一个与ATP结合位点相邻的次级结合位点,称为DFG外变构结合位点。格列卫和Nexavar最近在治疗慢性粒细胞白血病和肾细胞癌方面取得的成功,引起了人们对开发针对这一次级结合位点的其他激酶抑制剂的极大兴趣。在这里,我们对格列卫(R)、Nexavar和BIRB-796与各自的DFG外变构结合囊结合所需的重要和类似的相互作用进行了结构比较,并对各自对c-Abl、B-Raf和p38α的选择性进行了比较。通过直接从这些成功支架的片段中开发的8种额外的DFG外变构抑制剂的合成和评估,对它们的选择性特征进行了结构分析[4]。 |
| 细胞实验 |
将人胚肾 (HEK) 293 和 HeLa 细胞暴露于 0.5 M 山梨醇 30 分钟或 100 ng/mL EGF 10 分钟,然后在缓冲液 A(50 mM Tris-HCl,pH 7.5、1 mM EGTA、1 mM EDTA、1 mM 原钒酸钠、10 mM 氟化钠、50 mM β-甘油磷酸钠、5 mM 焦磷酸盐、0.27 M 蔗糖、0.1 mM 苯甲基磺酰氟、1% (v/v) Triton X-100) 加 0.1% (v/ v) 2-巯基乙醇和完全蛋白酶抑制剂混合物。在 4°C 下以 18,000×g 离心 5 分钟后,从裂解液中除去上清液。然后将它们快速冷冻在液氮中并保存在 -20°C 直至需要。必要时,将细胞在有或没有 10 μM SB 203580、10 μM PD 184352 或不同浓度的 Doramapimod 的情况下预孵育 1 小时,持续时间如图所示。
|
| 动物实验 |
Mice: The animals are 6- to 8-week-old athymic nude mice (BALB/c-nu/nu), weighing 18 to 24 g. BIRB 796 (Doramapimod) (10 mg/kg p.o., every 3 days×5) is given to the mice as a treatment. Every three days, the animal weights, the diameters of the two perpendicular tumors (A and B), and the estimated tumor volume (V) are all recorded.
Rats: Age-matched nontransgenic Sprague-Dawley (SD) rats (MDC) and male transgenic dTGRs (RCC Ltd) are used. There are two different protocols used. In protocol 2, rats from the untreated dTGR (n=15), dTGR+BIRB 796 (Doramapimod) (n=11) and SD (n=8 each group) groups are examined. Every week, a tail cuff is used to measure systolic blood pressure. In metabolic cages, 24-hour urine samples are taken from weeks 5 to 7. At week 7, serum is collected. Clinical standard assays are used to measure serum creatinine and cystatin C. Enzyme-linked immunosorbent assay is used to measure the albumin content of rat urine. Protocol 2 aims to concentrate on electrophysiological changes and mortality. Up to week 8, untreated dTGR (n = 10), dTGR+BIRB796 (n = 10), and SD (n = 10) rats are investigated. |
| 参考文献 | |
| 其他信息 |
Doramapimod is a member of the class of pyrazoles that is an immunomodulator used for treatment of rheumatoid arthritis, Crohn's disease and psoriasis. It has a role as an immunomodulator and an EC 2.7.11.24 (mitogen-activated protein kinase) inhibitor. It is a member of morpholines, a member of pyrazoles, a member of naphthalenes, a member of ureas and an aromatic ether.
Doramapimod is a P38 MAP kinase inhibitor. The p38 MAP kinase plays a crucial role in regulating the production of proinflammatory cytokines, such as tumor necrosis factor and interleukin-1. Blocking this kinase may offer an effective therapy for treating many inflammatory diseases. Here we report a new allosteric binding site for a diaryl urea class of highly potent and selective inhibitors against human p38 MAP kinase. The formation of this binding site requires a large conformational change not observed previously for any of the protein Ser/Thr kinases. This change is in the highly conserved Asp-Phe-Gly motif within the active site of the kinase. Solution studies demonstrate that this class of compounds has slow binding kinetics, consistent with the requirement for conformational change. Improving interactions in this allosteric pocket, as well as establishing binding interactions in the ATP pocket, enhanced the affinity of the inhibitors by 12,000-fold. One of the most potent compounds in this series, BIRB 796, has picomolar affinity for the kinase and low nanomolar inhibitory activity in cell culture.[1] We report on a series of N-pyrazole, N'-aryl ureas and their mode of binding to p38 mitogen activated protein kinase. Importantly, a key binding domain that is distinct from the adenosine 5'-triphoshate (ATP) binding site is exposed when the conserved activation loop, consisting in part of Asp168-Phe169-Gly170, adopts a conformation permitting lipophilic and hydrogen bonding interactions between this class of inhibitors and the protein. We describe the correlation of the structure-activity relationships and crystallographic structures of these inhibitors with p38. In addition, we incorporated another binding pharmacophore that forms a hydrogen bond at the ATP binding site. This modification affords significant improvements in binding, cellular, and in vivo potencies resulting in the selection of 45 (BIRB 796) as a clinical candidate for the treatment of inflammatory diseases.[2] The compound BIRB796 inhibits the stress-activated protein kinases p38alpha and p38beta and is undergoing clinical trials for the treatment of inflammatory diseases. Here we report that BIRB796 also inhibits the activity and the activation of SAPK3/p38gamma. This occurs at higher concentrations of BIRB796 than those that inhibit p38alpha and p38beta and at lower concentrations than those that inhibit the activation of JNK isoforms. We also show that at these concentrations, BIRB796 blocks the stress-induced phosphorylation of the scaffold protein SAP97, further establishing that this is a physiological substrate of SAPK3/p38gamma. Our results demonstrate that BIRB796, in combination with SB203580, a compound that inhibits p38alpha and p38beta, but not the other p38 isoforms, can be used to identify physiological substrates of SAPK3/p38gamma as well as those of p38alpha and p38beta.[3] We have previously shown that heat shock protein (Hsp) 27 or its upstream activator p38 mitogen-activated protein kinase (MAPK) confers resistance to bortezomib and dexamethasone (Dex) in multiple myeloma (MM) cells. This study examined anti-MM activity of a novel p38 MAPK inhibitor, BIRB 796, alone and in combination with conventional and novel therapeutic agents. BIRB 796 blocked baseline and bortezomib-triggered upregulation of p38 MAPK and Hsp27 phosphorylation, thereby enhancing cytotoxicity and caspase activation. The Hsp90 inhibitor 17-allylamino-17-demethoxy-geldanamycin (17-AAG) upregulated protein expression and phosphorylation of Hsp27; conversely, BIRB 796 inhibited this phosphorylation and enhanced 17-AAG-induced cytotoxicity. Importantly, BIRB 796 inhibited Hsp27 phosphorylation induced by 17-AAG plus bortezomib, thereby enhancing cytotoxicity. In bone marrow stromal cells (BMSC), BIRB 796 inhibited phosphorylation of p38 MAPK and secretion of interleukin-6 (IL-6) and vascular endothelial growth factor triggered by either tumour necrosis factor-alpha or tumour growth factor-beta1. BIRB 796 also inhibited IL-6 secretion induced in BMSCs by adherence to MM cells, thereby inhibiting tumour cell proliferation. These studies therefore suggest that BIRB 796 overcomes drug-resistance in the BM microenvironment, providing the framework for clinical trials of a p38 MAPK inhibitor, alone and in combination with bortezomib, Hsp90 inhibitor, or Dex, to improve patient outcome in MM.[4] We report on the structure-activity relationships (SAR) of 1-(5-tert-butyl-2-p-tolyl-2H-pyrazol-3-yl)-3-[4-(2-morpholin-4-yl-ethoxy)naphthalen-1-yl]urea (BIRB 796), an inhibitor of p38alpha MAP kinase which has advanced into human clinical trials for the treatment of autoimmune diseases. Thermal denaturation was used to establish molecular binding affinities for this class of p38alpha inhibitors. The tert-butyl group remains a critical binding element by occupying a lipophilic domain in the kinase which is exposed upon rearrangement of the activation loop. An aromatic ring attached to N-2 of the pyrazole nucleus provides important pi-CH(2) interactions with the kinase. The role of groups attached through an ethoxy group to the 4-position of the naphthalene and directed into the ATP-binding domain is elucidated. Pharmacophores with good hydrogen bonding potential, such as morpholine, pyridine, and imidazole, shift the melting temperature of p38alpha by 16-17 degrees C translating into K(d) values of 50-100 pM. Finally, we describe several compounds that potently inhibit TNF-alpha production when dosed orally in mice.[5] |
| 分子式 |
C31H37N5O3
|
|
|---|---|---|
| 分子量 |
527.66
|
|
| 精确质量 |
527.289
|
|
| 元素分析 |
C, 70.56; H, 7.07; N, 13.27; O, 9.10
|
|
| CAS号 |
285983-48-4
|
|
| 相关CAS号 |
|
|
| PubChem CID |
156422
|
|
| 外观&性状 |
White to gray solid powder
|
|
| 密度 |
1.2±0.1 g/cm3
|
|
| 沸点 |
631.6±55.0 °C at 760 mmHg
|
|
| 闪点 |
335.8±31.5 °C
|
|
| 蒸汽压 |
0.0±1.9 mmHg at 25°C
|
|
| 折射率 |
1.620
|
|
| LogP |
6.11
|
|
| tPSA |
80.65
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
5
|
|
| 可旋转键数目(RBC) |
8
|
|
| 重原子数目 |
39
|
|
| 分子复杂度/Complexity |
777
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
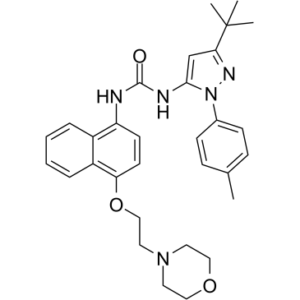
O1C([H])([H])C([H])([H])N(C([H])([H])C([H])([H])OC2=C([H])C([H])=C(C3=C([H])C([H])=C([H])C([H])=C23)N([H])C(N([H])C2=C([H])C(C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H])=NN2C2C([H])=C([H])C(C([H])([H])[H])=C([H])C=2[H])=O)C([H])([H])C1([H])[H]
|
|
| InChi Key |
MVCOAUNKQVWQHZ-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37)
|
|
| 化学名 |
1-[5-tert-butyl-2-(4-methylphenyl)pyrazol-3-yl]-3-[4-(2-morpholin-4-ylethoxy)naphthalen-1-yl]urea
|
|
| 别名 |
BIRB796; BIRB-796; Doramapimod; BIRB 796; BIRB0796; BIRB-0796
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (4.74 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL 澄清 DMSO 储备液加入900 μL 玉米油中,混合均匀。 配方 2 中的溶解度: ≥ 2.08 mg/mL (3.94 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清的DMSO储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 View More
配方 3 中的溶解度: 2.08 mg/mL (3.94 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 配方 4 中的溶解度: 30% PEG400+0.5% Tween80+5% Propylene glycol : 30mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.8952 mL | 9.4758 mL | 18.9516 mL | |
| 5 mM | 0.3790 mL | 1.8952 mL | 3.7903 mL | |
| 10 mM | 0.1895 mL | 0.9476 mL | 1.8952 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02211885 | Completed | Drug: 14C-BIRB 796 BS | Healthy | Boehringer Ingelheim | October 2002 | Phase 1 |
| NCT02211144 | Completed | Drug: BIBR 796 BS Drug: Placebo |
Healthy | Boehringer Ingelheim | March 2002 | Phase 1 |
| NCT02211157 | Completed | Drug: BIBR 796 BS Drug: Placebo |
Healthy | Boehringer Ingelheim | March 2000 | Phase 1 |
| NCT02209779 | Completed | Drug: BIBR 796 BS Drug: Placebo |
Arthritis, Rheumatoid | Boehringer Ingelheim | May 2001 | Phase 2 |
| NCT02209831 | Completed | Device: BIBR 796 BS Drug: Pantoprazole |
Healthy | Boehringer Ingelheim | November 2001 | Phase 1 |
|
 |