| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 靶点 |
LXRβ/liver X receptor β
|
|---|---|
| 体外研究 (In Vitro) |
在反式激活实验中,与全泛激动剂相比,XL041 (BMS-852927) 表现出 20% LXRα 和 88% LXRβ 活性。 XL041 在体外人全血内源性靶基因激活测定 (WBA) 中的 EC50 为 9 nM,活性为 26%,是一种非常强大的药物。据报道,BMS-852927 对 LXRα 和 LXRβ(分别为 19 和 12 nM)具有相当的结合亲和力 [1]。
当氟R3取代与2,6-二氯A环(15/BMS-852927)结合时,观察到非常强的hWBA活性,EC50值为9 nM(26%的疗效)。尽管15/BMS-852927具有相似的LXRα和LXRβ结合Ki值(分别为19和12 nM),但在激动剂试验中,与全泛激动剂相比,该化合物对LXRβ的疗效为88%,对LXRα的疗效仅为20%。在拮抗剂模式下测试时,15是一种强效的LXRα拮抗剂,IC50值为69 nM(抑制率为83%);而在高达10000 nM的LXRβ测定中没有观察到拮抗作用(支持信息)。通过2-Cl、6-F R1置换模式的进一步SAR研究,16个样本的hWBA效力为41 nM(33%)。在17中引入R3氯原子导致所有四种激动剂测定的疗效降低,hWBA EC50值为5 nM,略高于测定检测限值(16%)。R2处有氢的类似物18具有与宝石二甲基类似物12一致的体外活性。单甲基类似物19被鉴定为15的代谢产物,合成后的特征表明它也是一种强效的部分LXR激动剂[2]。 |
| 体内研究 (In Vivo) |
在有效剂量下,XL041 (BMS-852927) 在食蟹猴和小鼠中表现出非常积极的特征。给予C57BL/6J小鼠XL041 7天后,该系统的胆固醇流出以剂量依赖性方式得到有效刺激。在剂量为 3 mg/kg/天的组中观察到最高的外排率,比媒介物高 70%。 LDLR 敲除 (KO) 的小鼠也有类似的结果。在另一项为期 12 周的试验中,XL041 阻止了缺乏 LDLR 基因的小鼠动脉粥样硬化的进展。值得注意的是,增加巨噬细胞逆转胆固醇转移(RCT)(0.03-3 mg/kg/天)和抑制动脉粥样硬化(0.1-3 mg/kg/天)的剂量反应相当。是 LXR 激动剂影响疾病的关键潜在过程 [1]。
|
| 酶活实验 |
体外检测方法:[2]
配体结合分析。使用编码人RXRα和人LXRα或人LXRβ的杆状病毒共感染Sf9细胞。制备受感染的细胞裂解物,并将含有可溶性RXRα-LXR或RXRα-RXR¦异二聚体的上清液用于闪烁邻近配体结合分析,其中测试化合物与50 nM 3H-24,25-环氧胆固醇(NEN生命科学产品/Perkin Elmer)竞争结合的能力。1确定的Ki代表至少两个独立剂量反应实验的平均值。使用单位点竞争公式通过非线性回归分析确定每种化合物的结合亲和力,以确定IC50,其中:Y=底部+(顶部-底部)/(1+10X-logIC50)。然后计算Ki:Ki=IC50/(1+[配体浓度]/Kd配体)。对于该测定,通常配体浓度=50 nM,受体配体的Kd为200 nM,通过饱和结合测定。 瞬时转染报告检测。[2] LXR和LXR¦LXRE测定(同种型特异性)。对于激动剂试验,CV-1细胞与pCXM hLXRα或pCMX hLXRβ和LXREx1 tk-luc质粒共转染,并在含有5µL培养基和0.5%DMSO或试验激动剂的384孔板中以8000个细胞/孔的密度重新接种。以相同的方式进行2次LXR拮抗剂测定,但存在60 nM的激动剂Pan agonist A。基于在没有激动剂的情况下获得的值计算100%的抑制率。每个板的前两列含有泛激动剂A,以确定0%的抑制水平。将细胞孵育18-20小时,裂解并使用北极星HTS工作站(Applied BioSystems)测定萤光素酶活性。剂量反应曲线由浓度相差½对数单位的10点曲线生成。每个点代表来自32 384孔板的4孔数据的平均值。将该测定的数据拟合到以下方程中,可以求解EC50值:Y=底部+(顶部-底部)/(1+10((logEC50-X)*HillSlope))。EC50被定义为激动剂引发反应的浓度,该反应位于顶部(最大值)和底部(基线)值之间的一半。所表示的EC50值是至少2个独立实验的平均值。通过与泛激动剂A达到的最大反应进行比较来确定激动剂的相对疗效或%疗效(支持信息图1),该反应在每个剂量反应实验中单独测量。 LXR激动剂对巨噬细胞依赖性中性粒细胞减少途径影响的体外研究[1] 将巯基乙酸诱导的C57BL/6J小鼠腹腔巨噬细胞在DMEM-FBS中培养,并在无血清DMEM中与LXR激动剂或载体一起孵育20小时,然后在激动剂或赋形剂的持续存在下用20ng/mL LPS处理5小时。如补充实验程序的RNA制备和分析部分所述,确定了治疗对IL-23α和Mertk mRNA的影响。 |
| 细胞实验 |
用PBS洗涤每个烧瓶中的细胞,加入2 mL胰蛋白酶-EDTA(0.25%),在37℃、5%CO2下孵育5分钟。然后用力敲击烧瓶以破碎细胞聚集体。在加入8ml含有5%木炭/葡聚糖处理的FBS的DMEM后,将整个混合物转移到锥形管中。然后将细胞以1000rpm离心5分钟。将细胞沉淀重新悬浮在冷冻培养基(含20%血清和10%DMSO的DMEM)中,最终计数约为7 x 106个细胞/ml。将细胞悬浮液等分到15ml聚丙烯管中,每管5ml。将细胞放入-80℃的聚苯乙烯泡沫绝缘容器中过夜,缓慢冷冻。24小时后,将小瓶转移到超冷(-140℃)冰箱中长期储存。将冷冻保存的细胞小瓶在温水浴中快速解冻五分钟。将细胞合并,并在50ml锥形瓶中稀释至50ml。将解冻的细胞以1500rpm离心5分钟以收集细胞并丢弃上清液。然后将细胞重新悬浮在新鲜培养基II(DMEM,含有5%木炭/葡聚糖处理的FBS,1%青霉素/链霉素,100M非必需氨基酸、1 mM丙酮酸钠和2 mM L-谷氨酰胺),使用番石榴细胞计数器计数,并在相同的培养基中稀释至1.6 x 105个细胞/ml。将50微升细胞混合物加入到经白色组织培养处理的384孔板第1-23列的孔中,该板含有溶解在100%DMSO中的0.25l试验化合物。将50微升培养基II加入柱24中的孔中。将平板在37℃(5%CO2)下孵育24小时,然后向每个孔中加入5l Alamar Blue试剂。然后将板在37℃、5%CO2下再孵育两小时,然后在室温下孵育一小时。荧光读数为Ex525/Em598。在测量荧光之后,向每个孔中加入25l萤光素酶底物。将平板在室温下孵育15分钟,然后在PheraStar平板读数器上读取发光情况[1]。
|
| 动物实验 |
Cynomolgus Monkey Studies [1]
All studies were performed in male animals. In a PD study, animals were randomized into six treatment groups (n = 3/group) and dosed once daily with vehicle, 10 mg/kg/day T0901317, and 0.1, 0.3, 1, or 3 mg/kg/day BMS-852927 for 14 days. Blood RNA and plasma lipids were determined at baseline and days 1, 4, 7, and 14 of dosing for the PD study, and on days 1 and 7 for the liver TG MRS study (see below). In a cynomolgus monkey liver mRNA study conducted as part of a larger toxicology study, animals were randomized into four treatment groups (n = 5/group) and dosed daily for 28 days with vehicle, and 0.3, 3, or 30 mg/kg/day BMS-852927. A similar study was conducted with BMS-779788 in which animals were treated for 14 days with vehicle and 1, 10, or 30 mg/kg/day BMS-779788. Liver samples from both studies were taken at 24 hr after the final dose for compound concentration and mRNA determinations. All blood and liver mRNAs were quantitated as described in detail in the RNA preparation and analysis section of the Supplemental Experimental Procedures. To explore global effects of BMS-852927 on the transcriptome of multiple tissues, three animals each were treated with vehicle or 15 mg/kg/day BMS-852927 by oral gavage for 7 days. At 5–6 hr post-final dose, animals were sedated and then euthanized. Multiple tissues, including liver, spleen, aorta, right common carotid, bone marrow, and others, were harvested for transcriptome analysis using Affymetrix arrays as described in the Supplemental Experimental Procedures. In vivo MR spectroscopic measurements of liver TG were made using a Bruker 4.7T/40cm MRI system using Bruker Topspin software as described in detail in the Supplemental Experimental Procedures. Lipid analysis of the same region of interest in the central liver was performed on each of the primates after 7 days of oral dosing with either vehicle or BMS-852927 and compared to previously measured baseline values of the same liver region of interest. To determine the effect of BMS-852927 on sterol excretion, feces were collected at baseline and at the end of treatment in the 14 day PD study described above. Dried samples plus added standards were solubilized in NaOH-ethanol-water, and petroleum ether extracted material was analyzed for cholesterol by ultra-performance liquid chromatography. Mouse Studies [1] To study effects of LXR agonists on neutrophils, C57BL/6 mice pre-acclimated to oral dosing (n = 8/group) were randomly assigned to vehicle; 0.03, 0.1, 1, or 3 mg/kg/day BMS-852927; and 0.3 or 3 mg/kg/day GW3965 and dosed orally for 3 days. Following anesthesia with isoflurane, blood was collected by retro-orbital bleeding and analyzed for neutrophil levels using an Advia hematology instrument employing peroxidase staining. In an atherosclerosis prevention study, 8- to 10-week-old LDL receptor null mice fed a western diet were orally gavaged daily with vehicle, BMS-852927 (0.1, 1, or 3 mg/kg/day), or 10 mg/kg/day T0901317 for 12 weeks. At the end of treatment, mice were euthanized and atherosclerosis was quantitated en face in Oil Red O-stained aortas by image analysis. Lesion area was expressed as percent of total aortic area. To determine the effect of BMS-852927 on in vivo macrophage cholesterol efflux, C57Bl/6J mice (12–24 weeks of age, n = 3–4/group) were treated with vehicle or 0.03, 0.1, or 3 mg/kg/day BMS-852927 and dosed once daily for 7 days by oral gavage. Five hours post-final dose, animals were anesthetized and bovine serum albumin:[3H]-cholesterol particulate complexes were injected i.v. via the orbital plexus of non-fasted animals. Tail vein blood samples were collected from 5 to 150 min post-injection, and, following isolation by centrifugation, plasma radioactivity was determined by liquid scintillation counting. The time course of plasma radioactivity as a percentage of injected label was plotted, and the linear portion of the sterol reappearance phase following the rapid initial clearance was used to calculate initial macrophage cholesterol efflux rates using linear regression. Phase 1 Single, CV201-001, and Multiple, CV201-002, Ascending Dose Studies [1] Healthy men and women 18–45 years of age with a BMI of 18–30 kg/m2 were enrolled in randomized, double-blind, and placebo-controlled SAD and MAD studies. In each dose panel, eight subjects were randomized in a 3:1 ratio to receive BMS-852927 (n = 6) or matched placebo (n = 2). In the SAD study, subjects (mean age of 24.2 years and BMI of 24.1 kg/m2) were assigned to six sequential panels to receive a single dose of BMS-852927 (0.4, 2, 8, 20, 40, and 80 mg) or placebo. There were no deaths or SAEs in the SAD study; the most frequent treatment-emergent AE was hypertriglyceridemia reported in four (7.1%) subjects, all of whom were receiving BMS-852927 and were considered related to the study drug by the Investigator. In the MAD study, subjects were assigned to five sequential dose panels to receive BMS-852927 (0.2, 1, 2.5, 5, and 15 mg) or placebo once daily for 14 days. Exclusion criteria for the MAD study included MRI contraindications such as metal implants or prostheses. Phase 1b Study, CV201008 [1] Male and female patients between the ages of 18 and 75 years with primary hypercholesterolemia and with a BMI ≤40 kg/m2 were enrolled. Subjects were required to have been receiving a stable daily dose of a statin for ≥6 weeks with serum triglyceride levels at screening <400 mg/dL. Ninety-seven subjects were randomized and received study medication, 90 (92.8%) subjects completed the study, 7 (7.2%) subjects discontinued, 6 (6.2%) subjects discontinued due to AEs, and 1 (1.0%) discontinued due to other reason. Subjects received 0.25, 1, or 2.5 mg BMS-852927 or placebo once daily for 28 days and subjects remained on their pre-study statin treatment regimen throughout the study. Safety assessments were based on medical review of AE reports, the results of electrocardiograms, and the review of clinical laboratory tests, including fasting serum lipids. Refer to the Supplemental Experimental Procedures for more detail on study design and execution. The concentration of BMS-852927 in human plasma samples was determined using a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay as described in detail in the Supplemental Experimental Procedures. In vivo Methods [2] In vivo Pharmacokinetics in Mice [2] The pharmacokinetics were characterized in male C57BL6 mice (21.6-36 g). Mice received compound by oral gavage (fasted overnight) as a suspension in 0.75% CMC/0.1% Tween 80 in water. Blood samples (~0.2 mL) were obtained from 3 mice per time point at 0.25, 0.5, 1, 3, 6, 8 and 24 h post dose resulting in a composite pharmacokinetic profile (three blood sample were collected from each mouse). Blood samples were allowed to coagulate and centrifuged at 4 °C (1500-2000xg) to obtain serum. Serum samples were stored at -20 C until analysis by LC/MS/MS. Distribution Into Mice Brain [2] Distribution of 15 into the brain was studied following oral administration (10 mg/kg) to C57BL6 mice (N = 12). Brain and blood samples were collected at 1, 4, 8 and 24 h post dose (N = 3 animals per time point). Brain tissues were blotted dry, weighed, and homogenized (1:6 v/v) with 50% acetonitrile/water and centrifuged (10,000xg at 4°C for 10 min). Plasma samples were obtained from blood by centrifugation at 4°C (1500-2000xg). All samples were stored at -20°C until analysis by LC/MS/MS. Cynomolgus Monkey Single-dose Pharmacokinetics [2] The pharmacokinetics to evaluate clearance in cynomolgus monkeys (Table 2) was performed either by i.v. dosing of a single compound (e.g. BMS-852927) or i.v. dosing at 0.2 mg/kg in a cassette of 5 compounds that have different molecular weights. The i.v. infusion was done over 10 minutes (vehicle: 10% EtOH; 50% PEG400; 40% saline) two monkeys by similar procedures described below. The pharmacokinetics were evaluated in male cynomolgus monkeys in a crossover design (SI Table 1). Following an overnight fast, 3 animals (4.5 to 5.7 kg) received drug by IV infusion (1 mg/kg over 10 min) via a femoral vein and by oral gavage (3 mg/kg), with a 1-week washout between treatments. Serial blood samples (~0.3 mL) were collected from a femoral artery pre-dose and at 0.17 (IV only), 0.25, 0.5, 0.75, 1, 2, 4, 6, 8, 24, 48 and 72 h post dose, and centrifuged at 4 °C (1500-2000xg) to obtain plasma. Plasma samples were stored at -20 C until analysis by LC/MS/MS. Cynomolgus Monkey Pharmacodynamics Studies. [2] Male cynomolgus monkeys were obtained from the Bioculture Group and pair-housed for 3 weeks for acclimation and animals were subsequently transferred to single housing in standard non-human primate cages at the start of the study. Water and standard primate chow 37 were provided ad libitum. Food was provided daily in the amount of 120 grams. When fasting was required, food was removed at 3 PM and returned on the following day at 9 AM, 2 hours after dosing. For the 14 day PD studies, animals were randomized into treatment groups (N=3/group; 3- 6 kg) and received the following treatments at 7 am, q.d. for 14 days by oral gavage: vehicle (0.5% carboxymethyl cellulose and 2% Tween 80), 10 mg/kg/d T0901317, and 0.1, 0.3, 1, or 3 mg/kg/d BMS-852927 or 0.3, 1, 3, 10, 30 mpk mg/kg/d BMS-832878. An initial venous blood sample was obtained on the day prior to the start of dosing (day -1) for baseline RNA and lipid measurements, followed by samples taken on days 1, 4, 7, and 14 of dosing for the PD study. Blood samples for RNA and compound exposure determinations were collected at 5-6 hours post-dose, and those for plasma lipids were collected 24 hours post-dose. Plasma triglycerides were determined using a kit for triglycerides from Roche Diagnostics. The values reported are the mean. Statistical analysis was done by ANOVA, using Dunnett’s post-hoc test. The reported inductions of ABCG1 in the text were statistically significant compared to baseline levels by ANOVA, using Dunnett’s post-hoc test with p < 0.05 or better. |
| 药代性质 (ADME/PK) |
BMS-852927 has an oral bioavailability of 42% (mouse), 84% (monkey), and t1/2 of 12.4h (mouse), 9.9h (monkey).[2]
Agonists 13 and 15/BMS-852927 were nominated for further study because both compounds had robust LXRβ efficacy with low LXRα agonist efficacy (<20%), which was anticipated to improve the separation of desired efficacy from TG and LDL-C effects. In addition, 15/BMS-852927 was very potent in the hWBA. Analogues 13 and 15/BMS-852927 were not active in 16 nuclear hormone receptor agonist assays (>10 μM), except PXR with EC50 values of 3 μM (85% of full agonism) and 1 μM (108% of full agonism), respectively. When dosed in mice at 10 mg/kg, the Cmax coverage was high compared to the hWBA potency (Supporting Information). Given that LXR agonists could have deleterious effects in brain, as observed with LXR-623, the brain levels were measured and found to be low with 15 having a brain to plasma ratio of <0.05. In cynomolgus monkeys, 13 and 15 displayed good bioavailability, moderate clearance rates, and 10–12 h plasma half-lives[2]. While 15/BMS-852927 was considered the lead compound due to exceptional hWBA potency coupled with low LXRα efficacy (Table 3), both 13 and 15/BMS-852927 were studied in cynomolgus monkeys for 14 days to investigate the ABCG1 dose response to the lipid effects compared to those of 1. The agonists showed robust induction of the RCT target gene ABCG1 in plasma at drug concentrations that were predicted by the cynomolgus monkey WBA potency (1 cynoWBA EC50 = 310 nM (100%); 13 cynoWBA EC50 = 52 nM (29%); 15 cynoWBA EC50 = 5 nM (32%)). ABCA1 had shown variable vehicle effects in multiple cynomolgus monkey studies, precluding its use as a pharmacodynamic biomarker. Both 13 and 15 had improved TG profiles compared to 1. Fourteen days of dosing 1 at 10 mg/kg (200 nM plasma concentration at 5 h) caused a 6-fold ABCG1 induction in blood cells with TGs elevated 140% over baseline values (p < 0.05, ANOVA). After 14 days, the 1 and 3 mg/kg doses of 13 afforded 4- and 10-fold ABCG1 induction in blood cells with 85 and 310 nM plasma exposures, respectively. These doses yielded TGs of 2% and 58% above baseline (not significant). Comparatively the 0.1, 0.3, and 1 mg/kg doses of 15 provided 5 h plasma exposures of 7.5, 22, and 57 nM with 4.7-, 15-, and 11-fold ABCG1 induction on day 14. The TGs were elevated nonsignificantly 20, 8, and 10% over baseline, respectively. As anticipated, 15 provided robust ABCG1 induction at very low plasma drug concentrations, with little effect on plasma TGs. A full data set from this cynomolgus monkey study 15 is reported elsewhere [2]. |
| 参考文献 | |
| 其他信息 |
The development of LXR agonists for the treatment of coronary artery disease has been challenged by undesirable properties in animal models. Here we show the effects of an LXR agonist on lipid and lipoprotein metabolism and neutrophils in human subjects. BMS-852927, a novel LXRβ-selective compound, had favorable profiles in animal models with a wide therapeutic index in cynomolgus monkeys and mice. In healthy subjects and hypercholesterolemic patients, reverse cholesterol transport pathways were induced similarly to that in animal models. However, increased plasma and hepatic TG, plasma LDL-C, apoB, apoE, and CETP and decreased circulating neutrophils were also evident. Furthermore, similar increases in LDL-C were observed in normocholesterolemic subjects and statin-treated patients. The primate model markedly underestimated human lipogenic responses and did not predict human neutrophil effects. These studies demonstrate both beneficial and adverse LXR agonist clinical responses and emphasize the importance of further translational research in this area. [1]
Introducing a uniquely substituted phenyl sulfone into a series of biphenyl imidazole liver X receptor (LXR) agonists afforded a dramatic potency improvement for induction of ATP binding cassette transporters, ABCA1 and ABCG1, in human whole blood. The agonist series demonstrated robust LXRβ activity (>70%) with low partial LXRα agonist activity (<25%) in cell assays, providing a window between desired blood cell ABCG1 gene induction in cynomolgus monkeys and modest elevation of plasma triglycerides for agonist 15/BMS-852927. The addition of polarity to the phenyl sulfone also reduced binding to the plasma protein, human α-1-acid glycoprotein. Agonist 15/BMS-852927 was selected for clinical development based on the favorable combination of in vitro properties, excellent pharmacokinetic parameters, and a favorable lipid profile.[2] |
| 分子式 |
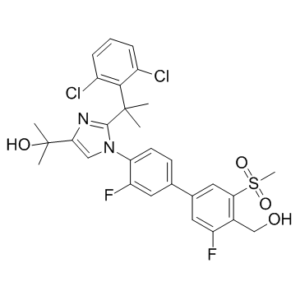
C29H28CL2F2N2O4S
|
|---|---|
| 分子量 |
609.511431694031
|
| 精确质量 |
608.111
|
| 元素分析 |
C, 57.15; H, 4.63; Cl, 11.63; F, 6.23; N, 4.60; O, 10.50; S, 5.26
|
| CAS号 |
1256918-39-4
|
| PubChem CID |
49787490
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
5.7
|
| tPSA |
101
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
40
|
| 分子复杂度/Complexity |
974
|
| 定义原子立体中心数目 |
0
|
| SMILES |
C(C1C(=CC=CC=1Cl)Cl)(C1=NC(C(O)(C)C)=CN1C1C=CC(C2C=C(F)C(CO)=C(S(=O)(=O)C)C=2)=CC=1F)(C)C
|
| InChi Key |
HNAJDMYOTDNOBK-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C29H28Cl2F2N2O4S/c1-28(2,26-19(30)7-6-8-20(26)31)27-34-25(29(3,4)37)14-35(27)23-10-9-16(11-22(23)33)17-12-21(32)18(15-36)24(13-17)40(5,38)39/h6-14,36-37H,15H2,1-5H3
|
| 化学名 |
2-(2-(2-(2,6-dichlorophenyl)propan-2-yl)-1-(3,3′-difluoro-4′-(hydroxymethyl)-5′-(methylsulfonyl)biphenyl-4-yl)-1H-imidazol-4-yl)propan-2-ol
|
| 别名 |
BMS-852927; BMS 852927; BMS852927; XL041; 1256918-39-4; BMS-852,927; XL-652; EXEL-04541041; H9649L8MZN; UNII-H9649L8MZN;XL-041; XL 041.
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~164.07 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (4.10 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (4.10 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (4.10 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.6407 mL | 8.2033 mL | 16.4066 mL | |
| 5 mM | 0.3281 mL | 1.6407 mL | 3.2813 mL | |
| 10 mM | 0.1641 mL | 0.8203 mL | 1.6407 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。