| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
CXCR4
|
|---|---|
| 体外研究 (In Vitro) |
TG-0054 是一种水溶性良好的抗血管生成药物,在治疗脉络膜新生血管方面具有潜在价值。在确定工艺参数对粒度和药物含量的影响后,制备了可通过 27 G 针头注射的球形微粒,平均直径为 7.6 μm,负载量为 10% w/w TG-0054,体外药物缓释至少 6 个月,残留有机溶剂含量低(~ 1 ppb/mg)。
|
| 体内研究 (In Vivo) |
在新西兰白兔玻璃体内注射微粒和药物溶液 3 个月后,对其体内药物输送情况进行了评估。3 个月时,微粒给药眼中的药物水平分别为 43.7 ± 16.2、243 ± 42.6、62.8 ± 22.6 μg/g 玻璃体、视网膜和脉络膜 RPE,与 1 个月时的水平相似。玻璃体内注射纯药物溶液导致给药眼中的药物量明显降低,1 个月时玻璃体、视网膜和脉络膜 RPE 中的药物水平分别为 0.8 ± 0.5、2.7 ± 2.8 和 4.9± 4.2 μg/g,3 个月时未检测到药物。尽管表面降解明显,但微粒在 6 个月的体外研究和 3 个月的体内研究期间仍保持其球形结构,1 个月和 3 个月的玻璃体颗粒保留率分别为 60% 和 27%。
|
| 细胞实验 |
将称量好的(5 至 10 毫克)载有 TG-0054 的微粒分散在 1 毫升含有 0.05% 叠氮化钠的磷酸盐缓冲盐水 (PBS,pH 7.4) 中。叠氮化钠可作为防腐剂,防止微粒制剂的微生物降解。将这些分散的微粒添加到透析袋 (7Spectra/por®,MWCO 25 kDa) 中,透析袋的一端用夹子预先密封。加入微粒分散液后,关闭透析袋的另一端。将装有微粒分散液的透析袋放入试管中的药物释放介质 (10 毫升 PBS,pH 7.4,含有 0.05% 叠氮化钠)。将试管在 37 °C 下孵育,同时以 200 rpm 的速度搅拌内容物。在 0.5 小时、1 小时、2 小时、4 小时、8 小时、16 小时、1 天、2 天以及此后每周等离散时间间隔内,将整个释放介质替换为保持在 37°C 的新鲜介质。使用紫外分光光度计分析在每个时间间隔移除的溶解介质,并测定释放介质中的 TG-0054 量。所有体外研究均重复进行三次。[1]
|
| 动物实验 |
The in vivo intravitreal delivery of TG-0054 from microparticle formulation was tested in male New Zealand white rabbits and compared with TG-0054 solution. Male New Zealand white rabbits weighing 2-3 kg were assigned to two groups. Group 1 (n = 6) received TG-0054-PLA microparticles intravitreally in one eye. Group 2 (n = 6) received TG-0054 solution intravitreally in both eyes. The rabbits were anaesthetized by intramuscular injection of ketamine : xyalazine mixture (50:10 v/v) in the hind limb of rabbits (400 μl/rabbit). Once the rabbits were in deep anesthesia, betadine solution was applied on eye surface and intravitreal injections were made using a 27G needle. Group 1 animals received 50 microliters of 300 mg microparticles/ml PBS (pH 7.4) [TG-0054-PLA microparticles with 10% drug loading; 15mg microparticles containing 1.5mg TG-0054/ 50μl] in the vitreal cavity of the right eye while the left eye was not dosed. Group 2 animals received 50 microliters of 20 mg TG-0054/ml PBS (pH 7.4) [1 mg/50 μl] in both eyes. After intravitreal injection, gentamicin ointment was applied at the injection site to prevent infections. The animals were regularly monitored for any abnormal signs. Three rabbits from each group were sacrificed at the end of 1 or 3 months post-dosing, in order to compare drug delivery between the formulations at the end of 1 and 3 months. Animals were euthanized by intravenous injection of sodium pentobarbital (150 mg/kg), and both eyes were enucleated and blood was collected. The eyes were immediately frozen in isopentane : dry ice bath and stored at −80 °C until further analysis.[1]
|
| 参考文献 |
|
| 其他信息 |
Burixafor Hydrobromide is the hydrobromide salt form of burixafor, an orally bioavailable inhibitor of CXC chemokine receptor 4 (CXCR4) with hematopoietic stem cell (HSC)-mobilization and chemosensitizing activities. Upon administration, burixafor binds to the chemokine receptor CXCR4, thereby preventing the binding of stromal cell-derived factor-1 (SDF-1 or CXCL12) to the CXCR4 receptor and subsequent receptor activation. This may induce the mobilization of hematopoietic stem and progenitor cells from the bone marrow into the peripheral circulation. Additionally, burixafor-mediated mobilization of disseminated tumor cells (DTCs) from the bone marrow into the blood may make these metastatic tumor cells more susceptible to the actions of chemotherapeutic agents. CXCR4, a chemokine receptor belonging to the G protein-coupled receptor (GPCR) gene family, plays an important role in chemotaxis and angiogenesis and is upregulated in several tumor cell types. CXCL12/CXCR4 interaction induces retention of hematopoietic cells in the bone marrow.
|
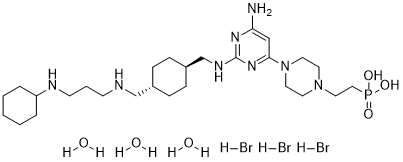
| 分子式 |
C27H52BRN8O3P
|
|---|---|
| 分子量 |
647.631345748901
|
| 精确质量 |
646.31
|
| 元素分析 |
C, 37.56; H, 7.00; Br, 27.76; N, 12.98; O, 11.12; P, 3.59
|
| CAS号 |
1191450-19-7
|
| 相关CAS号 |
1191450-19-7 (HBr); 1191448-17-5 (free acid)
|
| PubChem CID |
154575080
|
| 外观&性状 |
White to off-white solid powder
|
| tPSA |
152
|
| 氢键供体(HBD)数目 |
7
|
| 氢键受体(HBA)数目 |
11
|
| 可旋转键数目(RBC) |
14
|
| 重原子数目 |
40
|
| 分子复杂度/Complexity |
724
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
ZXUVXYNBMUFEMK-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C27H51N8O3P.BrH/c28-25-19-26(35-15-13-34(14-16-35)17-18-39(36,37)38)33-27(32-25)31-21-23-9-7-22(8-10-23)20-29-11-4-12-30-24-5-2-1-3-6-24;/h19,22-24,29-30H,1-18,20-21H2,(H2,36,37,38)(H3,28,31,32,33);1H
|
| 化学名 |
2-[4-[6-amino-2-[[4-[[3-(cyclohexylamino)propylamino]methyl]cyclohexyl]methylamino]pyrimidin-4-yl]piperazin-1-yl]ethylphosphonic acid;hydrobromide
|
| 别名 |
TG-0054; Burixafor; TG0054; Burixafor HBr; TG 0054; Burixafor HBr hydrate; Burixafor trihydrobromide trihydrate
|
| HS Tariff Code |
2934.99.03.00
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O: ~50 mg/mL (~65.0 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 20 mg/mL (26.01 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。
请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.5441 mL | 7.7205 mL | 15.4409 mL | |
| 5 mM | 0.3088 mL | 1.5441 mL | 3.0882 mL | |
| 10 mM | 0.1544 mL | 0.7720 mL | 1.5441 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02478125 | Terminated | Drug: Burixafor Hydrobromide Drug: Docetaxel |
Prostate Cancer | Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins |
July 2016 | Phase 1 |