| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
| 靶点 |
MIP-1α-CCR1 ( Ki = 1 nM ); RANTES-CCR1 ( Ki = 2.8 nM ); MCP-3-CCR1 ( Ki = 5.5 nM )
|
|
|---|---|---|
| 体外研究 (In Vitro) |
体外活性:BX471(也称为 ZK-811752)是一种新型口服非肽 CCR1(CC 趋化因子受体-1)拮抗剂,对人 CCR1 的 Ki 为 1 nM,可用于治疗慢性炎症性疾病。 BX471 对 CCR1 的选择性是 CCR2、CCR5 和 CXCR4 的 250 倍。 CCR1是治疗自身免疫性疾病的主要治疗靶点。与 28 G 蛋白偶联受体相比,BX 471 对 CCR1 的选择性高出 10,000 倍以上。竞争结合研究表明,BX 471 能够以高亲和力(K(i) 范围为 1 nm)取代 CCR1 配体巨噬细胞炎症蛋白-1α (MIP-1α)、RANTES 和单核细胞趋化蛋白-3 (MCP-3)至 5.5 nm)。 BX 471 是一种有效的功能拮抗剂,因为它能够抑制许多 CCR1 介导的效应,包括 Ca(2+) 动员、细胞外酸化率增加、CD11b 表达和白细胞迁移。此外,BX 471 能有效减少多发性硬化症大鼠实验性过敏性脑脊髓炎模型中的疾病。激酶测定:BX471(也称为 ZK-811752)是一种新型口服非肽 CCR1(CC 趋化因子受体-1)拮抗剂,对人 CCR1 的 Ki 为 1 nM,可能可用于治疗慢性炎症性疾病。 BX471 对 CCR1 的选择性是 CCR2、CCR5 和 CXCR4 的 250 倍。 CCR1是治疗自身免疫性疾病的主要治疗靶点。细胞测定:BX471 (0.1-10 μM) 在分离的血液单核细胞的剪切流中显示出对 RANTES 介导的和对 IL-1β 激活的微血管内皮的抗剪切粘附的剂量依赖性抑制。 BX471 还抑制 RANTES 介导的 T 淋巴细胞与活化内皮细胞的粘附。BX471 还能够以浓度依赖性方式取代 125I-MIP-1α/CCL3 与小鼠 CCR1 的结合,Ki 为 215±46 nM。增加 BX471 浓度可抑制人和小鼠 CCR1 中 MIP-1α/CCL3 诱导的 Ca2+ 瞬变,IC50 分别为 5.8±1 nM 和 198±7 nM。BX 471 是一种有效的功能拮抗剂,因为它能够抑制CCR1 介导的效应包括 Ca2+ 动员、细胞外酸化率增加、CD11b 表达和白细胞迁移。与 28 个 G 蛋白偶联受体相比,BX 471 对 CCR1 的选择性高出 10,000 倍以上。
|
|
| 体内研究 (In Vivo) |
BX471(20 mg/kg,皮下注射)在约 30 分钟内达到峰值血浆水平 9 μM,并在 2 小时后迅速下降至约 0.4 μM。 4 至 8 小时后,血浆药物水平降至 0.1 μM 或更低。用 20 mg/kg BX471 治疗 10 天的小鼠显示间质 CD45 阳性白细胞减少约 55%。 BX471 对外周血中 CCR5 阳性 CD8 细胞的数量具有临界显着影响。与载体对照相比,BX471 使 UUO 肾脏中 FSP1 阳性细胞的数量减少 65%。用 BX471 预处理可减少缺血再灌注损伤后肾脏中巨噬细胞和中性粒细胞的积累。BX 471(4 mg/kg,口服或静脉注射)是口服有效,狗体内生物利用度为 60%。此外,BX 471 能有效减少多发性硬化症大鼠实验性过敏性脑脊髓炎模型中的疾病。
|
|
| 酶活实验 |
趋化因子结合研究[1]
如前所述,通过过滤进行结合分析。使用终浓度约为0.1-0.2 nm的放射性标记趋化因子作为配体。使用每个测定点8000或300000个细胞表达人CCR1的HEK293细胞作为受体来源。在存在100nm未标记趋化因子的情况下测定非特异性结合。结合数据用计算机程序IGOR进行曲线拟合,以确定亲和力和位点数量。 胞浆Ca2+测量[1] 将表达人CCR1的HEK293细胞以80000个细胞/孔的速度铺在聚-d-赖氨酸涂覆的黑壁96孔板上,并培养过夜。然后,在Hanks的平衡盐溶液中,在37°C下用4μm Fluo-3(一种钙敏感的荧光染料)装载细胞60分钟,该溶液含有20 mm Hepes、3.2 mm氯化钙、1%胎牛血清、2.5 mm丙磺舒和0.04%普朗尼克酸。使用Denley洗涤器用测定缓冲液(Hanks平衡盐溶液,含有20mmHepes、2.5mm丙磺舒和0.1%牛血清白蛋白)轻轻洗涤细胞4次,去除多余的染料。在37°C下加入激动剂后,立即用FLIPR测量细胞内游离Ca2+浓度的变化。为了检测BX471的拮抗活性,在加入激动剂之前,用该化合物对细胞进行15分钟的预处理。根据方程式Ca2+=K D(F−F min)/(F max−F)计算细胞内Ca2+浓度(nm)(7)。K D是Fluo-3和Ca2+复合物的离解常数(Fluo-3为390 nm)。F是测量的荧光强度。F max是在0.1%triton X-100存在的情况下测定的最大荧光强度。F min是在0.1%曲拉通X-100加5 mmEGTA存在下测定的最小荧光强度。 BX471(也称为 ZK-811752)是一种新型口服非肽 CCR1(CC 趋化因子受体-1)拮抗剂,对人 CCR1 的 Ki 为 1 nM。它可能有助于治疗慢性炎症。与 CCR2、CCR5 和 CXCR4 相比,BX471 对 CCR1 的偏好程度高出 250 倍。在治疗自身免疫性疾病时,CCR1 是首要治疗靶点。 |
|
| 细胞实验 |
总之,在培养皿中培养至汇合的真皮微血管内皮细胞用 IL-1β (10 ng/mL) 刺激持续 12 小时,并在检测前立即与 RANTES (10 nM) 预孵育37°C 30 分钟。将板安装在带有×20和×40相衬物镜的Olympus IMT-2倒置显微镜的载物台上,并将它们组装为平行壁流室的下壁。将分离的人血单核细胞以 5×105 细胞/mL 的密度重悬于含有 0.5% 人血清白蛋白、10 mM HEPES、pH 7.4 的测定缓冲液 (HBSS) 中。在测定前不久添加 1 mM Mg2+ 和 1 mM Ca2+。将细胞悬浮液以 1.5 dyn/cm2 的速率灌注到流动室中五分钟,同时将其保持在 37°C 的加热块中进行测定。进行抑制实验的单核细胞首先与不同浓度 (0.1–10 μM) 的 Me2SO 对照或 BX471 在 37°C 下预孵育 10 分钟。以细胞/mm2 表示,5 分钟后,通过使用 JVC SR L 900 E 录像机和长镜头进行图像分析,在多个视野(每个实验至少 5 个)中量化牢固贴壁细胞的数量。集成JVC 3CCD 摄像机。原发性粘附,或单核细胞和内皮细胞之间的直接相互作用,是唯一被检查的粘附类型。
BX471 (0.1–10 μM) 以剂量依赖性方式抑制分离血液单核细胞剪切流中 IL-1β 激活的微血管内皮的抗剪切和 RANTES 介导的粘附。此外,BX471 还可抑制 T 细胞 RANTES 介导的与活化内皮细胞的粘附。 BX471 的 Ki 为 215±46 nM,还可以以浓度依赖性方式取代 125I-MIP-1α/CCL3 与小鼠 CCR1 的结合。 BX471 在人和小鼠 CCR1 中抑制 MIP-1α/CCL3 诱导的 Ca2+ 瞬变,随着化合物浓度的增加,IC50 值分别为 5.8±1 nM 和 198±7 nM。 BX 471 能够阻断多种 CCR1 介导的过程,例如白细胞迁移、细胞外酸化率增加、Ca2+ 动员和 CD11b 表达,使其成为强大的功能拮抗剂。 BX 471 对 CCR1 的选择性比 28 G 蛋白偶联受体高 10,000 倍以上。 外周血单个核细胞CD11b的表达[1] 如上所述测量全血测定中外周血单核细胞上表达的CD11b。简而言之,通过静脉穿刺将人全血收集到含有EDTA的2.5ml Vacutainer管中。血液保持在室温下,抽血后立即使用。全血样本(200μl)在37°C下用或不用1μmBX471预处理15分钟,然后用或不用100 nm MIP-1α再处理15分钟。通过加入1 ml冷磷酸盐缓冲盐溶液洗涤液终止反应。试管离心(200×g,4°C下7分钟),通过抽吸去除上清液。将细胞沉淀重新悬浮在冷磷酸盐缓冲盐溶液中,加入10μl 1mg/ml热聚集IgG,并在4°C下孵育试管10分钟。将抗体CD11b FITC(5μl)和CD14 PE(20μl)加入每个检测管中,在4°C下孵育20分钟。最后,加入1ml冰冷的磷酸盐缓冲盐溶液,如上所述将细胞造粒,并通过FACScan进行分析。 |
|
| 动物实验 |
|
|
| 药代性质 (ADME/PK) |
Pharmacokinetics of BX 471 in Dogs [1]
The oral bioavailability of BX 471 was examined in conscious dogs. BX 471 was administered to fasted male beagle dogs at 4 mg/kg in a vehicle of 40% cyclodextrin in saline by bolus intravenous injection via the cephalic vein or by oral gavage. The plasma samples were prepared, and compound concentrations in the plasma were determined by HPLC-MS. As shown in Fig.9, BX 471 reached peak plasma levels approximately 2 h after oral dosing and maintained measurable concentrations for up to 6 h. BX 471 exhibits a volume of distribution (0.5 l/kg) close to the volume of body water (0.6 l/kg), suggesting that the compound is confined primarily to the aqueous volume (Table III). Low clearance, 2 ml/min/kg (which represents less than 10% of the total liver blood flow) in the dog resulted in a moderate terminal half-life of 3 h (Fig. 9 and Table III). For dogs that were orally dosed, the half-life for BX 471 was approximately 3 h. Calculations of percent oral availability using area under curve measurements obtained from analysis using TOPFIT software indicated that BX 471 is an orally absorbed drug in fasted dogs with an oral bioavailability of approximately 60% (Fig.9 and Table III). |
|
| 毒性/毒理 (Toxicokinetics/TK) |
Effect of BX 471 on General Toxicity [1]
To demonstrate that CCR1 antagonism by BX 471 was not due to the cellular toxicity of the compound, THP-1- or CCR1-transfected HEK293 cells were treated with BX 471 at concentrations up to 10 μm for 24 h, and cellular toxicity was monitored by measuring WST-1 staining. No significant toxicity was observed (data not shown). The toxicity for BX 471 was further examined in vivo by a battery of serum diagnostic tests including hepatic and renal function tests and blood electrolytes on rabbits that had been dosed with BX 471 at 20 mg/kg/day for 30 days. The test results all fell within the normal range (data not shown). The results suggest that the inhibition of BX 471 on CCR1 activation was not due to cellular toxicity and that chronic treatment with the drug had no adverse effects on the normal physiology of the animals. |
|
| 参考文献 |
|
|
| 其他信息 |
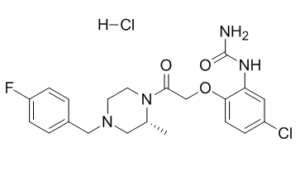
The CC chemokine receptor-1 (CCR1) is a prime therapeutic target for treating autoimmune diseases. Through high capacity screening followed by chemical optimization, we identified a novel non-peptide CCR1 antagonist, R-N-[5-chloro-2-[2-[4-[(4-fluorophenyl)methyl]-2-methyl-1-piperazinyl ]-2-oxoethoxy]phenyl]urea hydrochloric acid salt (BX 471). Competition binding studies revealed that BX 471 was able to displace the CCR1 ligands macrophage inflammatory protein-1alpha (MIP-1alpha), RANTES, and monocyte chemotactic protein-3 (MCP-3) with high affinity (K(i) ranged from 1 nm to 5.5 nm). BX 471 was a potent functional antagonist based on its ability to inhibit a number of CCR1-mediated effects including Ca(2+) mobilization, increase in extracellular acidification rate, CD11b expression, and leukocyte migration. BX 471 demonstrated a greater than 10,000-fold selectivity for CCR1 compared with 28 G-protein-coupled receptors. Pharmacokinetic studies demonstrated that BX 471 was orally active with a bioavailability of 60% in dogs. Furthermore, BX 471 effectively reduces disease in a rat experimental allergic encephalomyelitis model of multiple sclerosis. This study is the first to demonstrate that a non-peptide chemokine receptor antagonist is efficacious in an animal model of an autoimmune disease. In summary, we have identified a potent, selective, and orally available CCR1 antagonist that may be useful in the treatment of chronic inflammatory diseases. [1]
The expression of chemokines and their receptors is thought to contribute to leukocyte infiltration and progressive renal fibrosis after unilateral ureter obstruction (UUO). We hypothesized that blocking the chemokine receptor CCR1 using the nonpeptide antagonist BX471 could reduce leukocyte infiltration and renal fibrosis after UUO. UUO kidneys from BX471-treated mice (day 0-10 and day 6-10) revealed a 40-60% reduction of interstitial macrophage and lymphocyte infiltrate compared with controls. Treated mice also showed a marked reduction of CCR1 and CCR5 mRNA levels, and FACS analysis showed a comparable reduction of CD8+/CCR5+ T cells. Markers of renal fibrosis, such as interstitial fibroblasts, interstitial volume, mRNA and protein expression for collagen I, were all significantly reduced by BX471-treatment compared with vehicle controls. By contrast treatment was ineffective when the drug was supplied only from days 0 to 5. In summary, blockade of CCR1 substantially reduces cell accumulation and renal fibrosis after UUO. Most interestingly, late onset of treatment is also effective. We therefore conclude that CCR1 blockade may represent a new therapeutic strategy for reducing cellular infiltration and renal fibrosis as major factors in the progression to end-stage renal failure.[2] Neutrophils and macrophages rapidly infiltrate the kidney after renal ischemia-reperfusion injury, however specific molecular recruitment mechanisms have not been fully delineated for these cell types. Here we provide genetic and pharmacologic evidence supporting a positive role for the chemokine receptor CCR1 in macrophage and neutrophil infiltration in a 7 day mouse model of renal ischemia-reperfusion injury. By day 7, injured kidneys from mice lacking CCR1 contained 35% fewer neutrophils and 45% fewer macrophages than injured kidneys from wild-type control mice. Pretreatment of wild-type mice with the specific CCR1 antagonist BX471 also suppressed neutrophil and macrophage infiltration in the model. Injured kidneys from mice lacking CCR1 also had reduced content of the CCR1 ligands CCL3 (MIP-1alpha) and CCL5 (RANTES) compared with injured kidneys from wild-type controls, suggesting a leukocyte source for these inflammatory chemokines and existence of a CCR1-dependent positive feedback loop for leukocyte infiltration in the model. Local leukocyte proliferation and apoptosis were detected after injury, but were not dependent on CCR1. Also, the extent of necrotic and fibrotic damage and decline in renal function in injured kidneys was similar in wild-type and CCR1-deficient mice. Thus, CCR1 appears to regulate trafficking of macrophages and neutrophils to kidney in a mouse model of renal ischemia-reperfusion injury, however this activity does not appear to affect tissue injury.[3] Chemokines like RANTES appear to play a role in organ transplant rejection. Because RANTES is a potent agonist for the chemokine receptor CCR1, we examined whether the CCR1 receptor antagonist BX471 is efficacious in a rat heterotopic heart transplant rejection model. Treatment of animals with BX471 and a subtherapeutic dose of cyclosporin (2.5 mg/kg), which is by itself ineffective in prolonging transplant rejection, is much more efficacious in prolonging transplantation rejection than animals treated with either cyclosporin or BX471 alone. We have examined the mechanism of action of the CCR1 antagonist in in vitro flow assays over microvascular endothelium and have discovered that the antagonist blocks the firm adhesion of monocytes triggered by RANTES on inflamed endothelium. Together, these data demonstrate a significant role for CCR1 in allograft rejection. [4] |
| 分子式 |
C21H25CL2FN4O3
|
|
|---|---|---|
| 分子量 |
471.35
|
|
| 精确质量 |
470.129
|
|
| 元素分析 |
C, 53.51; H, 5.35; Cl, 15.04; F, 4.03; N, 11.89; O, 10.18
|
|
| CAS号 |
288262-96-4
|
|
| 相关CAS号 |
BX471; 217645-70-0
|
|
| PubChem CID |
5311124
|
|
| 外观&性状 |
White to yellow solid powder
|
|
| LogP |
4.346
|
|
| tPSA |
88.89
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
5
|
|
| 可旋转键数目(RBC) |
6
|
|
| 重原子数目 |
31
|
|
| 分子复杂度/Complexity |
591
|
|
| 定义原子立体中心数目 |
1
|
|
| SMILES |
O=C(N)NC1=CC(Cl)=CC=C1OCC(N2[C@H](C)CN(CC3=CC=C(F)C=C3)CC2)=O.[H]Cl
|
|
| InChi Key |
FRUCNQBAWUHKLS-PFEQFJNWSA-N
|
|
| InChi Code |
InChI=1S/C21H24ClFN4O3.ClH/c1-14-11-26(12-15-2-5-17(23)6-3-15)8-9-27(14)20(28)13-30-19-7-4-16(22)10-18(19)25-21(24)29;/h2-7,10,14H,8-9,11-13H2,1H3,(H3,24,25,29);1H/t14-;/m1./s1
|
|
| 化学名 |
[5-chloro-2-[2-[(2R)-4-[(4-fluorophenyl)methyl]-2-methylpiperazin-1-yl]-2-oxoethoxy]phenyl]urea;hydrochloride
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 3 mg/mL (6.36 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 30.0 mg/mL 澄清的 DMSO 储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL 生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 3 mg/mL (6.36 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 30.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 3 mg/mL (6.36 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1216 mL | 10.6078 mL | 21.2157 mL | |
| 5 mM | 0.4243 mL | 2.1216 mL | 4.2431 mL | |
| 10 mM | 0.2122 mL | 1.0608 mL | 2.1216 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT00185341 | Completed | Drug: Placebo Drug: CCR1-Antagonist (BAY86-5047, ZK811752) |
Endometriosis | Bayer | February 2005 | Phase 2 |
 |
|---|
 |
 |