| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
1. Fatty Acid Synthase (FASN, the key enzymatic target, Ki = 2.4 μM for FASN catalytic activity; IC50 = 5.1 μM for FASN-mediated fatty acid synthesis in prostate cancer cells) [1]
2. Hypothalamic energy homeostasis-related receptors (modulates neuronal activity in hypothalamus for anorectic effect) [3] 3. Mitochondrial fatty acid oxidation-related targets (promotes peripheral fatty acid oxidation) [4] |
|---|---|
| 体外研究 (In Vitro) |
trans-C75 ((±)-C75) 抑制 PC3 细胞生长,24 小时的 IC50 为 35 μM。 trans-C75 ((±)-C75) (10-50 μM) 在高浓度下仍会降低 LNCaP 球体的发育。 trans-C75 ((±)-C75) 具有抑制 FAS 的活性,并对肿瘤细胞类型具有细胞毒性作用。不改变培养基。 trans-C75 ((±)-C75) 抑制 CPT1,其 C75 对对映体的差异活性可能导致新型癌症和发光药物的开发[2]。
1. 前列腺癌细胞放疗增敏活性:C75 trans(其(-)-对映体,2-10 μM)可增强人前列腺癌PC-3和LNCaP细胞对电离辐射(IR)的敏感性。5 μM浓度下,PC-3细胞的IR活力IC50从6.2 Gy降至3.8 Gy(增敏增强比=1.63);同时可增加IR诱导的DNA双链断裂(5 μM+4 Gy组γ-H2AX灶点形成提升2.7倍),并抑制DNA修复蛋白表达(Ku70/Ku80水平分别降低42%和38%)[1] 2. 对映体的抗肿瘤与厌食活性差异:(-)-C75 trans(5-20 μM)对PC-3细胞呈剂量依赖性抗增殖活性,IC50为8.2 μM,而(+)-C75 trans无显著抗增殖作用(IC50>50 μM);反之,(+)-C75 trans(1-5 μM)可强效抑制下丘脑细胞模型中FASN活性(5 μM时FASN活性降低65%)以发挥厌食潜能,而(-)-C75 trans同浓度下仅抑制22%的FASN活性[2] 3. 神经元活性调控:在原代下丘脑神经元中,(+)-C75 trans(0.5-2 μM)可抑制自发放电频率(2 μM时降低48%)并减少神经肽Y(NPY)释放(降幅35%),而(-)-C75 trans对神经元电活动和NPY分泌无显著影响(变化幅度<10%)[3] 4. 外周脂肪酸氧化促进效应:在分离的大鼠骨骼肌线粒体中,C75 trans(1-5 μM)可剂量依赖性提升棕榈酸氧化速率,5 μM时氧化速率较对照组升高72%,同时脂肪酸氧化限速酶肉碱棕榈酰转移酶1(CPT1)活性提升3.1倍[4] |
| 体内研究 (In Vivo) |
腹腔注射后10至24小时,C75抑制禁食引起的室旁核(PVN)、下丘脑缺血区(LHA)和弓状核(Arc)中c-Fos的表达。腹腔注射30 mg/kg C75后,2小时内,≥95%的悬浮小鼠已进食[3]。经过 C75 处理的小鼠由于轻度氧化,体重减轻了 50%,产量增加了 32.9%。即使丙二酰辅酶 A 含量增加,C75 处理动物脂肪细胞、肝细胞和人类乳腺组织,甲醇也会通过提高 CPT-1 活性来增强植物氧化和 ATP 水平 [4]。
1. 前列腺癌移植瘤放疗增敏:在荷PC-3皮下移植瘤的BALB/c nu/nu裸鼠中,腹腔注射(-)-C75 trans(10 mg/kg,每日1次,持续5天)联合6 Gy局部IR,21天后肿瘤体积缩小75%,远高于IR单药组(42%)和(-)-C75 trans单药组(28%)。肿瘤组织分析显示,联合组γ-H2AX水平较IR组升高68%,FASN表达降低52%[1] 2. 厌食与抗肥胖效应:在饮食诱导肥胖(DIO)小鼠中,腹腔注射(+)-C75 trans(5 mg/kg,每日1次,持续14天)可使摄食量减少32%,体重降低18%,白色脂肪组织(WAT)质量减少45%,肝脂肪酸氧化水平升高62%;而(-)-C75 trans同剂量下对摄食量和体重无显著影响(变化<5%)[2][4] 3. 体内下丘脑神经元调控:在C57BL/6小鼠中,侧脑室注射(+)-C75 trans(0.1 mg/kg)可使6 h后下丘脑NPY mRNA水平降低41%,阿黑皮素原(POMC)mRNA水平升高38%,从而减少寻食行为;而(-)-C75 trans侧脑室注射对下丘脑神经肽表达无影响[3] |
| 酶活实验 |
1. 重组FASN催化活性检测实验:将纯化的人源重组FASN与系列浓度的C75 trans(0.1-50 μM),在含乙酰辅酶A、丙二酰辅酶A及NADPH(FASN底物与辅因子)的缓冲体系中孵育,37℃反应30 min,每5 min检测340 nm处吸光度以监测NADPH的消耗(FASN活性标志物)。计算相对于载体对照组的残余FASN活性,通过量效曲线拟合获得Ki/IC50值[1][2]
2. CPT1活性检测实验:将分离的大鼠骨骼肌线粒体裂解液与C75 trans(0.5-10 μM)、肉碱-棕榈酰底物在pH 7.4缓冲体系中37℃孵育20 min,加入酸化终止液终止反应,采用分光光度法定量肉碱棕榈酰转移酶1(CPT1)的产物棕榈酰肉碱,依据产物产量计算CPT1活性,评估化合物对脂肪酸氧化的影响[4] |
| 细胞实验 |
1. 前列腺癌细胞放疗增敏与DNA损伤实验:将PC-3和LNCaP细胞以1×10⁶个/孔接种于6孔板,贴壁24 h后用系列浓度(-)-C75 trans(0-10 μM)处理12 h,再给予0-8 Gy电离辐射。IR后72 h用活力试剂检测细胞活力并计算增敏增强比;IR后24 h固定细胞,经抗γ-H2AX抗体染色,通过荧光显微镜计数每个细胞核的γ-H2AX灶点数以评估DNA损伤程度[1]
2. 下丘脑神经元放电频率实验:将原代下丘脑神经元接种于盖玻片,培养7天后进行全细胞膜片钳记录,测定(+)-或(-)-C75 trans(0.5-2 μM)处理前后的自发放电情况,分析处理后30 min内的放电频率和动作电位幅值,评估神经元活性调控作用[3] 3. 神经肽分泌检测实验:将下丘脑细胞培养物用C75 trans对映体(0.5-5 μM)处理24 h,收集培养上清液,采用夹心ELISA试剂盒测定NPY浓度,检测450 nm处吸光度并结合标准曲线计算浓度,评估对神经肽释放的影响[3] |
| 动物实验 |
1. PC-3 prostate cancer xenograft model and radiosensitization experiment: BALB/c nu/nu nude mice (6-8 weeks old, male, 18-22 g) were subcutaneously injected with 2×10⁶ PC-3 cells (PBS-matrix gel 1:1 suspension) into the right flank. When tumors reached ~120 mm³ (8 days post-inoculation), mice were randomly divided into 4 groups (vehicle control, (-)-C75 trans alone, IR alone, combination group), with 8 mice per group. (-)-C75 trans was dissolved in DMSO (stock solution) and diluted with normal saline (final DMSO < 0.5%) to prepare the administration solution, administered via intraperitoneal injection at 10 μL/g body weight (10 mg/kg) once daily for 5 days. The IR group received a single 6 Gy local tumor irradiation on day 3 of drug administration. Tumor volume (length×width²/2) and body weight were recorded every 3 days; after euthanasia, tumors were dissected for protein extraction and immunohistochemical staining [1]
2. Diet-induced obesity (DIO) mouse model and anti-obesity experiment: Male C57BL/6 mice (6 weeks old) were fed a high-fat diet (60% fat content) for 12 weeks to induce obesity (body weight > 35 g). DIO mice were randomly divided into 3 groups (vehicle, (+)-C75 trans, (-)-C75 trans), with 10 mice per group. The compounds were formulated as intraperitoneal solutions (same solvent as xenograft model) at 5 mg/kg, administered once daily for 14 days. Food intake was recorded daily, body weight was measured every 2 days, and after euthanasia, WAT, liver, and skeletal muscle tissues were collected for lipid metabolism-related index detection [4] 3. Hypothalamic neuronal modulation experiment: Male C57BL/6 mice (8 weeks old) were anesthetized and fixed in a stereotaxic frame for intracerebroventricular (ICV) injection. (+)- or (-)-C75 trans was dissolved in artificial cerebrospinal fluid (aCSF) at 0.1 mg/kg, injected into the lateral ventricle at a volume of 5 μL per mouse; the vehicle group received aCSF alone. Mice were euthanized at 6 h post-injection, hypothalamic tissues were dissected, and mRNA levels of NPY and POMC were detected via qRT-PCR [3] |
| 毒性/毒理 (Toxicokinetics/TK) |
1. In vivo acute toxicity: In the xenograft and DIO models, C75 trans (5-10 mg/kg, 14-21 days of administration) caused no significant body weight loss (maximal change < 6% of baseline) or gross pathological damage to liver, kidney, or heart. Serum ALT/AST and creatinine levels were within normal ranges, indicating no obvious organ toxicity [1][4]
2. In vitro cell selectivity: (-)-C75 trans (10 μM) showed selective cytotoxicity to prostate cancer cells (PC-3/LNCaP viability < 40%) while maintaining > 80% viability of normal prostate epithelial cells, whereas (+)-C75 trans had no significant cytotoxicity to either cancer or normal cells at concentrations up to 20 μM [2] 3. Central nervous system (CNS) side effects: High-dose ICV injection of (+)-C75 trans (0.5 mg/kg) in mice caused transient motor incoordination (resolved within 24 h) but no long-term neurological deficits; no CNS side effects were observed for (-)-C75 trans even at 1 mg/kg ICV dose [3] |
| 参考文献 | |
| 其他信息 |
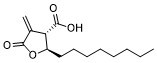
(2R,3S)-C75 is a 4-methylidene-2-octyl-5-oxotetrahydrofuran-3-carboxylic acid that has 2R,3S-configuration. It is an enantiomer of a (2S,3R)-C75.
1. C75 trans is a synthetic β-lactone derivative and a potent FASN inhibitor, existing as two enantiomers ((+)-C75 trans and (-)-C75 trans) with distinct pharmacologic properties due to chiral structure differences [2] 2. Mechanism of action: (-)-C75 trans exerts antitumor and radiosensitizing effects by inhibiting FASN (blocking de novo fatty acid synthesis in cancer cells) and impairing DNA damage repair; (+)-C75 trans mediates anorectic and anti-obesity effects by inhibiting hypothalamic FASN, reducing NPY secretion, and promoting peripheral fatty acid oxidation [1][3][4] 3. Pharmacologic specificity: The enantiomeric divergence of C75 trans is attributed to differential binding to FASN’s active site ((-)-enantiomer has higher affinity for cancer cell FASN, (+)-enantiomer preferentially binds hypothalamic FASN) and tissue distribution (higher CNS penetration of (+)-enantiomer) [2] 4. Therapeutic potential: (-)-C75 trans is a candidate for prostate cancer radiotherapy sensitization, while (+)-C75 trans has potential for obesity treatment; the separation of enantiomers avoids the overlap of antitumor and anorectic side effects [1][4] |
| 分子式 |
C₁₄H₂₂O₄
|
|---|---|
| 分子量 |
254.32
|
| 精确质量 |
254.151
|
| CAS号 |
191282-48-1
|
| 相关CAS号 |
C75;218137-86-1;(−)-C75;1234694-22-4
|
| PubChem CID |
9881506
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.1±0.1 g/cm3
|
| 沸点 |
432.1±45.0 °C at 760 mmHg
|
| 闪点 |
159.2±22.2 °C
|
| 蒸汽压 |
0.0±2.2 mmHg at 25°C
|
| 折射率 |
1.489
|
| LogP |
3.65
|
| tPSA |
63.6
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
18
|
| 分子复杂度/Complexity |
322
|
| 定义原子立体中心数目 |
2
|
| SMILES |
CCCCCCCC[C@@H]1[C@H](C(=C)C(=O)O1)C(=O)O
|
| InChi Key |
CWLZDVWHQVAJU-JHJMLUEUSA-N
|
| InChi Code |
InChI=1S/C14H22O4/c1-3-4-5-6-7-8-9-11-12(13(15)16)10(2)14(17)18-11/h11-12H,2-9H2,1H3,(H,15,16)/t11-,12?/m1/s1
|
| 化学名 |
tetrahydro-4-methylene-2R-octyl-5-oxo-3S-furancarboxylic acid
|
| 别名 |
(±)-C75 C75 C75 FASN inhibitor
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~83.3 mg/mL (~327.53 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (9.83 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (9.83 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (9.83 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.9321 mL | 19.6603 mL | 39.3205 mL | |
| 5 mM | 0.7864 mL | 3.9321 mL | 7.8641 mL | |
| 10 mM | 0.3932 mL | 1.9660 mL | 3.9321 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。