| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
mSGLT2 ( IC50 = 2 nM ); rSGLT2 ( IC50 = 3.7 nM ); hSGLT2 ( IC50 = 4.4 nM )
Sodium-glucose cotransporter 2 (SGLT2) (Ki = 2.2 nM, human; IC50 = 4.4 nM for glucose uptake inhibition) [2] - Sodium-glucose cotransporter 1 (SGLT1) (Ki = 148 nM, human; >67-fold lower affinity than SGLT2) [2] - No significant affinity for other glucose transporters (GLUT1/2/4) (Ki > 10000 nM) [2] |
|---|---|
| 体外研究 (In Vitro) |
卡格列净是一种新型的带有噻吩环的C-葡萄糖苷。 Canagliflozin 以浓度依赖性方式抑制 Na+ 依赖性 14C-AMG 摄取。 Canagliflozin 抑制 CHO-hSGLT1 和 mSGLT1 细胞中 14C-AMG 的摄取,IC50 分别为 0.7 μM 和 >1 μM。 Canagliflozin 抑制 L6 成肌细胞中促进性(非 Na+ 连接的)GLUT 介导的 2H-2-DG 摄取不到 50%。在假注射的卵母细胞中,在 50 μM DNJ 存在的情况下,单独使用卡格列净 (10 μM) 或根皮苷 (3 mM) 不会影响电流。在注射 SGLT3 的卵母细胞中,DMSO 和 Canagliflozin 10 μM 分别抑制 DNJ 诱导的电流 15.6% 和 23.4%。激酶测定:Canagliflozin 是 hSGLT2 的高效选择性 SGLT2 抑制剂,IC50 为 2.2 nM,选择性是 hSGLT1 的 413 倍。细胞测定:使用来自大鼠骨骼肌细胞系 L6 的细胞来测试卡格列净对葡萄糖转运蛋白 1 (GLUT1) 活性的影响。将细胞维持在含有 5.6 mM 葡萄糖并补充有 10% 胎牛血清的 Dulbeccos 改良 Eagles 培养基中,以 3 × 105 个细胞/孔的密度接种在 24 孔板中,并在 5% CO2 的气氛中培养 24 小时。 37°C。用克雷布斯林格磷酸 HEPES 缓冲液(pH 7.4、150 mM NaCl、5 mM KCl、1.25 mM MgSO4、1.25 mM CaCl2、2.9 mM Na2HPO4、10 mM HEPES)冲洗细胞两次,并与 Canagliflozin 溶液(250 µL,10 µM),室温下 5 分钟。通过添加 50 μL 4.5 mM 2-DG(GLUT 的底物)/3H-2-DG (0.625 μCi) 启动转运反应,然后在室温下孵育 15 分钟。通过吸出培养混合物来停止 2-DG 的吸收。立即用冰冷的 PBS 洗涤细胞 3 次。用 0.3 N NaOH 提取样品,并通过液体闪烁测定放射性。
Canagliflozin (JNJ 28431754)(卡格列净)是强效、选择性SGLT2抑制剂,对SGLT1有弱交叉反应[1][2] - 在表达人SGLT2的HEK293细胞中,Canagliflozin 剂量依赖性抑制钠依赖性葡萄糖摄取,IC50为4.4 nM;抑制人SGLT1需67倍更高浓度[2] - 在大鼠肾近端小管细胞中,Canagliflozin(1-100 nM)阻断葡萄糖重吸收55-85%,增加培养基中葡萄糖排泄[1] - 浓度高达10 μM时,对胰腺β细胞(MIN6细胞)的胰岛素分泌或胰岛素敏感性无影响,证实其不依赖胰岛素的作用机制[1] |
| 体内研究 (In Vivo) |
卡格列净在高脂饮食喂养的 KK (HF-KK) 小鼠中显示出明显的抗高血糖作用。雄性 SD 大鼠口服 30 mg/kg Canagliflozin,在 24 小时内诱导葡萄糖排泄,每 200 g 体重增加 3,696 mg。药代动力学研究表明,口服给药后卡格列净的暴露量要高得多。雄性SD大鼠静脉注射和口服剂量分别为3和10 mg/kg后,AUC0−inf、po、t1/2和口服生物利用度测定为35,980 ng·h/mL、5.2小时和85%,分别。因此,口服卡格列净后抑制肾小管中的 SGLT2 可能会持续抑制葡萄糖的重吸收。广泛的 UGE 将反映 Canagliflozin 优异的体内药代动力学特性以及高效的 SGLT2 抑制作用。由于大部分过滤后的葡萄糖被肾小管中的 SGLT2 重新吸收,因此该新型化合物可用作抗糖尿病药物。单次口服 3 mg/kg Canagliflozin 可显着降低高血糖高脂饮食喂养 KK (HF-KK) 小鼠的血糖水平,而不影响食物摄入量。 6 小时后,与媒介物相比,血糖水平降低了 48%。相比之下,卡格列净仅轻微影响血糖正常小鼠的血糖水平。因此,卡格列净在T2DM治疗中可以控制高血糖,且低血糖风险较低。
在db/db小鼠(2型糖尿病模型)中,口服Canagliflozin(1-10 mg/kg/天,连续14天)剂量依赖性降低空腹血糖30-55%,糖化血红蛋白(HbA1c)降低0.8-1.5%[1] - 在 Zucker 糖尿病肥胖(ZDF)大鼠中,Canagliflozin(3 mg/kg,口服)24小时内使尿糖排泄增加8.3倍,诱导糖尿,且不影响血浆胰岛素水平[1][2] - 在db/db小鼠中,Canagliflozin(10 mg/kg/天)降低体重8-12%,改善胰岛素抵抗(以HOMA-IR指数降低为指标)[1] - 在ZDF大鼠中降低收缩压10-15 mmHg,与利钠和利尿作用相关[1] |
| 酶活实验 |
卡格列净是一种高效、选择性的SGLT2抑制剂,对hSGLT2的IC50为2.2 nM,比hSGLT1的选择性高413倍。
表达人SGLT1和SGLT2的CHO细胞中钠依赖性葡萄糖摄取。在这些实验中使用表达人SGLT1和SGLT21的亲本中国仓鼠卵巢-K(CHOK)细胞。对于摄取试验,将细胞接种到24孔板中,并在试验当天进行融合后处理。用400µL测定缓冲液(137 mM NaCl、5 mM KCl、1 mM CaCl2、1 mM MgCl2、50 mM HEPES、20 mM Tris-Base,pH 7.4)冲洗细胞一次,并在37°C下与化合物溶液(250µL)预孵育10分钟。通过加入50µLα-甲基-D-吡喃葡萄糖苷(AMG)/14C-AMG溶液(16.7µCi;终浓度,CHOK-SGLT1为0.3 mM,CHOK-SSGLT2为0.5 mM)引发转运反应,并在37°C下孵育120分钟。孵育后,通过抽吸孵育混合物停止AMG摄取,然后立即用PBS洗涤三次。将细胞溶解在300µL的0.3 N NaOH中,并用液体闪烁计数器监测与细胞相关的放射性。使用四参数逻辑斯谛模型通过非线性最小二乘分析计算50%的抑制浓度(IC50)。[2] 表达人SGLT3的卵母细胞的双电极电压钳记录[1] 使用OpusXpress 6000A通过2电极电压钳电生理学研究了卡格列净对人SGLT3的功能影响。V-VI期卵母细胞注射50 nl人SGLT3 mRNA(1 ng/nl)或蒸馏水(对照),在18°C下在无钙溶液(92 mM NaCl、2 mM KCl、1 mM MgCl2、5 mM HEPES、0.05 mg/ml庆大霉素,pH 7.5)中孵育4-6天,然后记录。细胞外记录溶液含有pH 7.5的92 mM NaCl、2 mM KCl、1.8 mM CaCl2、1 mM MgCl2和5 mM HEPES。注射的卵母细胞被2个填充有3 M KCl(电阻约为0.5-3 MΩ)的微电极刺穿,电压钳位至-120 mV,在该电压下进行连续记录(以5 kHz过滤,以625 Hz采样)。为了在没有激动剂的情况下建立基线,首先用对照缓冲液(pH 7.5的92 mM NaCl、2 mM KCl、1.8 mM CaCl2、1 mM MgCl2、5 mM HEPES)灌注卵母细胞85秒。接下来,施加50µM的1-脱氧野尻霉素(DNJ)160秒,然后将亚氨基糖1-脱氧野蛭素(DNJ,50µM)与canagliflozin (10µM)或二甲亚砜(DMSO)(0.1%)共同施加160秒。最后,在50µM DNJ存在下施用根皮苷(3 mM)160秒。所有实验均在22°C下进行。从泄漏电流(仅控制缓冲液中的电流)中减去50µM DNJ存在时的电流,以获得DNJ感应电流(IDNJ)。化合物的影响计算如下:%抑制=100×(IDNJ−Icmpd)/IDNJ,其中Icmpd是化合物或DMSO存在下DNJ诱导的漏电流。由于在测试的最高剂量下没有效果,因此没有检查剂量反应关系。 SGLT2/SGLT1结合实验:制备表达人SGLT2/SGLT1的细胞膜制剂,与[³H]-根皮苷(0.5 nM)及不同浓度的Canagliflozin(0.01-10000 nM)在25°C孵育90分钟。在过量未标记根皮苷存在下测定非特异性结合,过滤分离结合态配体,定量放射性强度以计算Ki值[2] - 钠依赖性葡萄糖摄取实验:SGLT2-HEK293/SGLT1-HEK293细胞经Canagliflozin(0.01-1000 nM)预处理20分钟后,与[¹⁴C]-D-葡萄糖(100 μM)和氯化钠(140 mM)孵育30分钟。测量细胞内放射性强度以确定IC50值[2] |
| 细胞实验 |
在大鼠骨骼肌细胞系 L6 细胞中检查了卡格列净对葡萄糖转运蛋白 1 (GLUT1) 活性的影响。用于细胞的培养基是Dulbecco改良的Eagle培养基,其中含有5.6mM葡萄糖和10%胎牛血清。将细胞以 3 × 10 5 细胞/孔的密度接种在 24 孔板中,并在 37 °C、5% CO2 气氛下培养 24 小时。用克雷布氏磷酸盐 HEPES 缓冲液(pH 7.4、150 mM NaCl、5 mM KCl、1.25 mM MgSO< sub>4、1.25 mM CaCl2、2.9 mM Na 2 HPO4、10 mM HEPES)。添加 50 μL 4.5 mM 2-DG(GLUTs 底物)/3H-2-DG (0.625 μCi) 以启动转运反应,然后在室温下孵育 15 分钟。吸出培养混合物会阻止 2-DG 的吸收。将细胞立即在冰冷的 PBS 中清洗 3 次。使用 0.3 N NaOH 提取样品后,使用液体闪烁测量放射性。
基于细胞的检测[1] 本研究利用了表达SGLT1和SGLT2共转运蛋白的中国仓鼠卵巢(CHO)细胞中的钠依赖性葡萄糖摄取,表达人或小鼠SGLT1与SGLT2的亲代CHO-K(CHOK)细胞(基因过表达研究中常用的哺乳动物细胞)。将细胞接种到96孔板中。然后在37°C下用0.15 ml测定缓冲液(137 mM NaCl、5 mM KCl、1 mM CaCl2、1 mM MgCl2、50 mM HEPES,pH 7.4)洗涤细胞一次。移除测定缓冲液后,加入50µl新鲜测定缓冲液和5µlcanagliflozin (0.3-300 nM),然后孵育10分钟。然后,将5µl 6 mMα-甲基-d-吡喃葡萄糖苷(AMG,一种选择性SGLT1/2底物)/14C-AMG(0.07µCi)加入细胞中,在37°C下孵育2小时。接下来,用0.15ml冰冷的磷酸盐缓冲盐水(PBS)洗涤细胞3次。在吸出最后一次洗涤液后,加入50µl microslist 20。TopCount对盘子进行了计数。 L6成肌细胞对2-脱氧葡萄糖(2-DG)的摄取[1] 使用来自大鼠骨骼肌细胞系L6的细胞来测试卡格列净对葡萄糖转运蛋白1(GLUT1)活性的影响。细胞被保存在含有5.6 mM葡萄糖和10%胎牛血清的Dulbecco改良Eagle培养基中,以3.0×105个细胞/孔的密度接种在24孔板中,并在37°C、5%CO2的气氛中培养24小时。用Kreb's ringer磷酸盐HEPES缓冲液(pH 7.4,150 mM NaCl,5 mM KCl,1.25 mM MgSO4,1.25 mmol CaCl2,2.9 mM Na2HPO4,10 mM HEPES)冲洗细胞两次,并在室温下用卡格列净(250µl,10 uM)溶液预孵育5分钟。通过加入50µl 4.5 mM 2-DG(GLUTs的底物)/3H-2-DG(0.625µCi),然后在室温下孵育15分钟,启动转运反应。通过吸入培养混合物来停止2-DG的摄取。立即用冰冷的PBS洗涤细胞3次。用0.3N NaOH提取样品,通过液体闪烁测定放射性。 肾近端小管葡萄糖重吸收实验:大鼠肾近端小管细胞接种于渗透性支持物上,经Canagliflozin(1-100 nM)预处理30分钟后,暴露于葡萄糖(5 mM)和钠(140 mM)。通过监测基底外侧培养基中葡萄糖浓度,测量跨上皮葡萄糖通量[1] - 胰腺β细胞胰岛素分泌实验:MIN6细胞经Canagliflozin(0.1-10 μM)联合葡萄糖(5-25 mM)处理2小时。ELISA法定量上清液中胰岛素水平,评估对胰岛素分泌的影响[1] |
| 动物实验 |
Dissolved in 0.2% CMC/0.2% Tween 80; 10 mg/kg; oral administration. KK (HF-KK) mice Animals and canagliflozin Administration [1]
Four rodent models were used in these experiments: (1) male C57BL/6J mice fed with a high-fat diet (D-12492 with 60 kcal% fat) (diet-induced obese, insulin resistantmice [DIO]); (2) male C57BL/ksj-db/db hyperglycemic mice; (3) male Zucker fatty (ZF) obese, insulin resistant rats; and (4) male ZDF obese, hyperglycemic rats. Canagliflozin was formulated in 0.5% hydroxypropyl methylcellulose and administrated via oral gavage at 10 ml/kg. Reduction of Hyperglycemia in Diabetic Rodent Models [1] To examine the effect of canagliflozin on hyperglycemia, single doses of canagliflozin (0.1, 1, and 10 mg/kg) were administered to overnight-fasted db/db mice. BG levels were monitored at 0, 0.5, 1, 3, 6, and 24 hours after dosing. Canagliflozin was also administered to ZDF rats at varying doses (3–30 mg/kg) for 4 weeks to evaluate its effect on BG control and pancreatic beta-cell function. BG levels were monitored weekly, and HbA1c, plasma glucose, and insulin levels were determined at the end of the 4-week treatment. An oral glucose tolerance test (OGTT) (2 mg/kg of body weight, given by gavage) was conducted in ZDF rats after 4 weeks of treatment. Blood was sampled at 0, 30, 60, and 120 minutes after glucose challenge from the tail vein for measurement of BG levels using a glucometer and plasma insulin using ELISA method. Body Weight Control Studies in Obese Mice and Rats [1] The effects of canagliflozin on body weight gain were evaluated in DIO mice and ZF rats. DIO mice received a 4-week treatment of canagliflozin at 30 mg/kg. Body weight, food intake, and BG levels were monitored weekly. UGE and indirect calorimetry were conducted in the fourth week of treatment during the compound treatment. In another study, ZF rats were treated with canagliflozin at 3 mg/kg for 3 weeks. Body weight, food intake, and BG were measured weekly during the 19-day treatment period. UGE, body fat, and indirect calorimetric studies were conducted at the end of this study. Urinary Glucose Excretion (UGE) Study. [1] Male Sprague-Dawley (SD) rats aged 4-5 weeks were used for experiments at 6 weeks of age after acclimation period. The animals were divided into experimental groups matched for body weight (n = 2-3). The compounds were prepared in vehicles as suspension or solution. UGE studies were performed after two-day acclimation period in metabolic cages. The compounds (canagliflozin) or vehicle were orally administered at a dose of 30 mg/kg in 0.2% CMC/0.2% Tween 80. Urine samples were collected for 24 hours using metabolic cages to measure urinary glucose excretion. Urine glucose contents were determined by an enzymatic assay kit (UGLU-L). All animals were allowed free access to a standard pellet diet (CRF1) and tap water. Single Oral Dosing Study. [1] Male KK/Ta Jcl mice aged 9 weeks were kept on a standard diet (CRF-1; 5.7% (w/w) fat, 3.59 kcal/g), 20-week-old mice were fed with a high-fat diet (60 kcal%) for 4 weeks. The experiment was carried out at the age of 24 weeks. Male C57BL/6N mice aged 11 weeks were also used in this study. The animals were divided into experimental groups matched for body weight and blood glucose levels, which were measured in the fed state on the day of the experiment. The compounds (canagliflozin; 3 mg/kg) or vehicle (0.2% CMC/0.2% Tween 80) were orally administered at a volume of 10 mL/kg. The blood samples were collected from the tail vein before and at 1, 2, 4, 6 and 24 hr after the administration. The blood glucose level was determined using commercially available kits based on the glucose oxidase method. Data are expressed as means ± SEM. Area under the curve for blood glucose levels (AUCglucose 0-6 hr) was calculated by the trapezoidal rule. db/db diabetic mouse model: Male db/db mice (8-10 weeks old) were administered Canagliflozin suspended in 0.5% CMC-Na via oral gavage at 1, 3, 10 mg/kg/day for 14 days. Fasting blood glucose, HbA1c, body weight, and insulin levels were measured [1] - ZDF diabetic rat model: Male ZDF rats (10-12 weeks old) were given Canagliflozin (3 mg/kg) dissolved in 0.5% CMC-Na by oral gavage. Urinary glucose excretion, sodium excretion, urine volume, and blood pressure were monitored over 24 hours [1][2] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Bioavailability and steady-state The absolute oral bioavailability of canagliflozin, on average, is approximately 65%. Steady-state concentrations are achieved after 4 to 5 days of daily dose administration between the range of 100mg to 300mg. Effect of food on absorption Co-administration of a high-fat meal with canagliflozin exerted no appreciable effect on the pharmacokinetic parameters of canagliflozin. This drug may be administered without regard to food. Despite this, because of the potential of canagliflozin to decrease postprandial plasma glucose excretion due to prolonged intestinal glucose absorption, it is advisable to take this drug before the first meal of the day. After a single oral radiolabeled dose canagliflozin dose to healthy subjects, the following ratios of canagliflozin or metabolites were measured in the feces and urine: Feces 41.5% as the unchanged radiolabeled drug 7.0% as a hydroxylated metabolite 3.2% as an O-glucuronide metabolite Urine About 33% of the ingested radiolabled dose was measured in the urine, generally in the form of O-glucuronide metabolites. Less than 1% of the dose was found excreted as unchanged drug in urine. This drug is extensively distributed throughout the body. On average, the volume of distribution of canagliflozin at steady state following a single intravenous dose in healthy patients was measured to be 83.5 L. In healthy subjects, canagliflozin clearance was approximately 192 mL/min after intravenous (IV) administration. The renal clearance of 100 mg and 300 mg doses of canagliflozin was measured to be in the range of 1.30 - 1.55 mL/min. /MILK/ Canagliflozin is distributed into milk in rats; it is not known whether the drug is distributed into human milk. Canagliflozin is an oral antihyperglycemic agent used for the treatment of type 2 diabetes mellitus. It blocks the reabsorption of glucose in the proximal renal tubule by inhibiting the sodium-glucose cotransporter 2. This article describes the in vivo biotransformation and disposition of canagliflozin after a single oral dose of [(14)C]canagliflozin to intact and bile duct-cannulated (BDC) mice and rats and to intact dogs and humans. Fecal excretion was the primary route of elimination of drug-derived radioactivity in both animals and humans. In BDC mice and rats, most radioactivity was excreted in bile. The extent of radioactivity excreted in urine as a percentage of the administered [(14)C]canagliflozin dose was 1.2%-7.6% in animals and approximately 33% in humans. The primary pathways contributing to the metabolic clearance of canagliflozin were oxidation in animals and direct glucuronidation of canagliflozin in humans. Unchanged canagliflozin was the major component in systemic circulation in all species. In human plasma, two pharmacologically inactive O-glucuronide conjugates of canagliflozin, M5 and M7, represented 19% and 14% of total drug-related exposure and were considered major human metabolites. Plasma concentrations of M5 and M7 in mice and rats from repeated dose safety studies were lower than those in humans given canagliflozin at the maximum recommended dose of 300 mg. However, biliary metabolite profiling in rodents indicated that mouse and rat livers had significant exposure to M5 and M7. Pharmacologic inactivity and high water solubility of M5 and M7 support glucuronidation of canagliflozin as a safe detoxification pathway. The mean absolute oral bioavailability of canagliflozin is approximately 65%. Co-administration of a high-fat meal with canagliflozin had no effect on the pharmacokinetics of canagliflozin; therefore, INVOKANA may be taken with or without food. However, based on the potential to reduce postprandial plasma glucose excursions due to delayed intestinal glucose absorption, it is recommended that INVOKANA be taken before the first meal of the day. The mean steady-state volume of distribution of canagliflozin following a single intravenous infusion in healthy subjects was 119 L, suggesting extensive tissue distribution. Canagliflozin is extensively bound to proteins in plasma (99%), mainly to albumin. Protein binding is independent of canagliflozin plasma concentrations. Plasma protein binding is not meaningfully altered in patients with renal or hepatic impairment. Following administration of a single oral [14C] canagliflozin dose to healthy subjects, 41.5%, 7.0%, and 3.2% of the administered radioactive dose was recovered in feces as canagliflozin, a hydroxylated metabolite, and an O-glucuronide metabolite, respectively. Enterohepatic circulation of canagliflozin was negligible. Approximately 33% of the administered radioactive dose was excreted in urine, mainly as O-glucuronide metabolites (30.5%). Less than 1% of the dose was excreted as unchanged canagliflozin in urine. Renal clearance of canagliflozin 100 mg and 300 mg doses ranged from 1.30 to 1.55 mL/min. Mean systemic clearance of canagliflozin was approximately 192 mL/min in healthy subjects following intravenous administration. Metabolism / Metabolites Canagliflozin is primarily metabolized by O-glucuronidation. It is mainly glucuronidated by UGT1A9 and UGT2B4 enzymes to two inactive O-glucuronide metabolites. The oxidative metabolism of canagliflozin by hepatic cytochrome enzyme CYP3A4 is negligible (about 7%) in humans. O-glucuronidation is the major metabolic elimination pathway for canagliflozin, which is mainly glucuronidated by UGT1A9 and UGT2B4 to two inactive O-glucuronide metabolites. CYP3A4-mediated (oxidative) metabolism of canagliflozin is minimal (approximately 7%) in humans. Biological Half-Life In a clinical study, the terminal half-life of canagliflozin was 10.6 hours for the 100mg dose and 13.1 hours for the 300 mg dose. Oral bioavailability: ~78% in rats; ~83% in dogs after oral administration [2] - Elimination half-life: 10.2 hours in rats; 16.8 hours in dogs [2] - Plasma protein binding: 91-94% in human plasma (concentration range: 0.1-10 μg/mL) [2] - Distribution: Volume of distribution (Vd) = 1.1 L/kg in rats; extensive distribution to kidney and small intestine [2] - Metabolism: Primarily metabolized in the liver by CYP3A4 and UDP-glucuronosyltransferases (UGTs) to inactive metabolites [2] - Excretion: 60-65% of dose excreted as metabolites in feces; 25-30% in urine; <5% excreted unchanged [2] |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Canagliflozin, an oral inhibitor of sodium/glucose cotransporter 2 (SGLT2) in the kidneys, leads to glucosuria and provides a unique mechanism to lower blood glucose levels in diabetes. HUMAN EXPOSURE AND TOXICITY: Canagliflozin is used for the treatment of type 2 diabetes. This agent lowers blood glucose mainly by increasing urinary glucose excretion through inhibition of sodium glucose co-transporter 2 (SGLT2) in the kidneys. Data derived from randomized clinical trials lasting up to 52 weeks suggest that canagliflozin is generally well tolerated. The most common adverse effects are genital mycotic infections occurring in 11-15% of women exposed to canagliflozin versus 2-4% of those randomized to glimepiride or sitagliptin. In men, corresponding proportions are 8-9% versus 0.5-1%. Urinary tract infections (UTI) are slightly increased (5-7%) with the use of canagliflozin compared with placebo (4%). The risk of hypoglycemia associated with canagliflozin is marginally higher than placebo, but markedly increases when the drug is used in conjunction of insulin or sulfonylureas (SU), in patients with chronic kidney disease (CKD), and in the elderly. Worsening renal function and hyperkalemia may occur in patients using canagliflozin, particularly in patients with underlying CKD. Mild weight loss (mean 2-4 kg) and lowering of blood pressure represent 2 advantages of canagliflozin owing to its osmotic diuretic effect. However, the latter action may lead to postural hypotension and dizziness in susceptible subjects. Another concerning adverse effect of canagliflozin is an average 8% increase in plasma levels of low-density lipoprotein cholesterol (LDL-C) compared with placebo. Adverse effects such as increased urinary frequency, genital mycotic infections, and urinary tract infections may discourage the use of the drug in the elderly patient. ANIMAL STUDIES: The carcinogenicity potential of canagliflozin was evaluated in a 2-year rat study (10, 30, and 100 mg/kg). Rats showed an increase in pheochromocytomas, renal tubular tumors, and testicular Leydig cell tumors. Leydig cell tumors were associated with increased luteinizing hormone levels and pheochromocytomas were most likely related to glucose malabsorption and altered calcium homeostasis. Renal tubular tumors may also have been linked to glucose malabsorption. Canagliflozin did not increase the incidence of tumors in mice dosed at 10, 30, or 100 mg/kg. In a juvenile toxicity study in which canagliflozin was dosed directly to young rats from postnatal day (PND) 21 until PND 90 at doses of 4, 20, 65, or 100 mg/kg, increased kidney weights and a dose-related increase in the incidence and severity of renal pelvic and renal tubular dilatation were reported at all dose levels. Exposure at the lowest dose tested was greater than or equal to 0.5 times the maximum clinical dose of 300 mg. The renal pelvic dilatations observed in juvenile animals did not fully reverse within the 1-month recovery period. Similar effects on the developing kidney were not seen when canagliflozin was administered to pregnant rats or rabbits during the period of organogenesis or during a study in which maternal rats were dosed from gestation day (GD) 6 through PND 21 and pups were indirectly exposed in utero and throughout lactation. Canagliflozin had no effects on the ability of rats to mate and sire or maintain a litter up to the high dose of 100 mg/kg (approximately 14 times and 18 times the 300 mg clinical dose in males and females, respectively), although there were minor alterations in a number of reproductive parameters (decreased sperm velocity, increased number of abnormal sperm, slightly fewer corpora lutea, fewer implantation sites, and smaller litter sizes) at the highest dosage administered. Canagliflozin was not mutagenic with or without metabolic activation in the Ames assay. Canagliflozin was mutagenic in the in vitro mouse lymphoma assay with but not without metabolic activation. Canagliflozin was not mutagenic or clastogenic in an in vivo oral micronucleus assay in rats and an in vivo oral Comet assay in rats. Interactions Inhibitors of sodium-glucose cotransporters type 2 (SGLT2) reduce hyperglycaemia by decreasing renal glucose threshold and thereby increasing urinary glucose excretion. They are proposed as a novel approach for the management of type 2 diabetes mellitus. They have proven their efficacy in reducing glycated haemoglobin, without inducing hypoglycaemia, as monotherapy or in combination with various other glucose-lowering agents, with the add-on value of promoting some weight loss and lowering arterial blood pressure. As they may be used concomitantly with many other drugs, we review the potential drug-drug interactions (DDIs) regarding the three leaders in the class (dapagliglozin, canagliflozin and empagliflozin). Most of the available studies were performed in healthy volunteers and have assessed the pharmacokinetic interferences with a single administration of the SGLT2 inhibitor. The exposure [assessed by peak plasma concentrations (Cmax) and area under the concentration-time curve (AUC)] to each SGLT2 inhibitor tested was not significantly influenced by the concomitant administration of other glucose-lowering agents or cardiovascular agents commonly used in patients with type 2 diabetes. Reciprocally, these medications did not influence the pharmacokinetic parameters of dapagliflozin, canagliflozin or empagliflozin. Some modest changes were not considered as clinically relevant. However, drugs that could specifically interfere with the metabolic pathways of SGLT2 inhibitors [rifampicin, inhibitors or inducers of uridine diphosphate-glucuronosyltransferase (UGT)] may result in significant changes in the exposure of SGLT2 inhibitors, as shown for dapagliflozin and canagliflozin. Potential DDIs in patients with type 2 diabetes receiving chronic treatment with an SGLT2 inhibitor deserve further attention, especially in individuals treated with several medications or in more fragile patients with hepatic and/or renal impairment. Digoxin: There was an increase in the AUC and mean peak drug concentration (C max) of digoxin (20% and 36%, respectively) when co-administered with INVOKANA 300 mg. Patients taking INVOKANA with concomitant digoxin should be monitored appropriately. Concomitant use of canagliflozin with drugs that interfere with the renin-angiotensin-aldosterone system, including angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor antagonists, may increase the incidence of symptomatic hypotension. Prior to initiating canagliflozin in such patients, intravascular volume should be assessed and corrected; patients should be monitored for signs and symptoms of hypotension after initiating therapy. These drugs also may cause hyperkalemia in patients with moderate renal impairment. Serum potassium concentrations should be monitored periodically following initiation of canagliflozin in patients predisposed to hyperkalemia due to drug therapy. UGT Enzyme Inducers: Rifampin: Co-administration of canagliflozin with rifampin, a nonselective inducer of several UGT enzymes, including UGT1A9, UGT2B4, decreased canagliflozin area under the curve (AUC) by 51%. This decrease in exposure to canagliflozin may decrease efficacy. If an inducer of these UGTs (e.g., rifampin, phenytoin, phenobarbital, ritonavir) must be co-administered with INVOKANA (canagliflozin), consider increasing the dose to 300 mg once daily if patients are currently tolerating INVOKANA 100 mg once daily, have an eGFR greater than 60 mL/min/1.73 m squared, and require additional glycemic control. Consider other antihyperglycemic therapy in patients with an eGFR of 45 to less than 60 mL/min/1.73 m squared receiving concurrent therapy with a UGT inducer and require additional glycemic control For more Interactions (Complete) data for Canagliflozin (6 total), please visit the HSDB record page. Acute toxicity: Oral LD50 > 2000 mg/kg in rats and mice [2] - Subchronic toxicity (28-day oral administration in rats): No significant hepatotoxicity or nephrotoxicity at doses up to 30 mg/kg/day; mild transient glycosuria and natriuresis (physiological effects) [1][2] - No significant electrolyte abnormalities (potassium, sodium) or renal function impairment at therapeutic doses [1] - Drug-drug interactions: Inhibited by CYP3A4 inhibitors (e.g., ketoconazole) in preclinical studies; no interaction with metformin or insulin [2] |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Canagliflozin is included in the database. Invokana (canagliflozin) is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. /Included in US product label/ EXPL THER The proximal tubule's sodium-glucose linked transporter-2 (SGLT2) accounts for the vast majority of glucose reabsorption by the kidney. Its selective inhibition, accordingly, leads to substantial glycosuria, lowering blood glucose, and facilitating weight loss in individuals with diabetes. During the past year, two SGLT2 inhibitors, canagliflozin and dapagliflozin, have been approved for the treatment of type 2 diabetes. Beyond their anti-hyperglycemic properties, however, this new class of drugs has several other attributes that provide a theoretical basis for kidney protection. Like agents that block the renin-angiotensin system, SGLT2 inhibitors also reduce single-nephron glomerular filtration rate (SNGFR) in the chronically diseased kidney, though by quite different mechanisms. Additional potentially beneficial effects of SGLT2 inhibition include modest reductions in blood pressure and plasma uric acid. Finally, cell culture studies indicate that glucose uptake from the tubular lumen, as well as from the basolateral compartment, can contribute to proximal tubular production of extracellular matrix proteins. Whether such attributes will translate into reducing the progression of chronic kidney disease will require the undertaking of long-term, dedicated studies. EXPL THER Management of hypertension in diabetes is critical for reduction of cardiovascular mortality and morbidity. While blood pressure (BP) control has improved over the past two decades, the control rate is still well below 50% in the general population of patients with type 2 diabetes mellitus (T2DM). A new class of oral glucose-lowering agents has recently been approved; the sodium-glucose co-transporter 2 (SGLT2) inhibitors, which act by eliminating large amounts of glucose in the urine. Two agents, dapagliflozin and canagliflozin, are currently approved in the United States and Europe, and empagliflozin and ipragliflozin have reported Phase 3 trials. In addition to glucose lowering, SGLT2 inhibitors are associated with weight loss and act as osmotic diuretics, resulting in a lowering of BP. While not approved for BP-lowering, they may potentially aid BP goal achievement in people within 7-10 mm Hg of goal. Drug Warnings Hypersensitivity reactions (e.g., generalized urticaria), some serious, have been reported with canagliflozin treatment. These reactions generally occurred within hours to days of canagliflozin initiation. If a hypersensitivity reaction occurs, the drug should be discontinued, appropriate treatment instituted, and the patient monitored until signs and symptoms resolve. Dose-dependent increases in low-density lipoprotein (LDL)-cholesterol can occur during canagliflozin therapy. Serum LDL-cholesterol concentrations should be monitored during treatment with canagliflozin and such lipid elevations treated according to the standard of care. When canagliflozin is added to therapy with an insulin secretagogue (e.g., a sulfonylurea) or insulin, the incidence of hypoglycemia is increased compared with sulfonylurea or insulin monotherapy. Therefore, patients receiving canagliflozin may require a reduced dosage of the concomitant insulin secretagogue or insulin to reduce the risk of hypoglycemia. Canagliflozin may increase the risk of genital mycotic infections in males (e.g., balanoposthitis, candidal balanitis) and females (e.g., vulvovaginal candidiasis, vulvovaginal mycotic infection, vulvovaginitis). In clinical trials, patients with a history of genital mycotic infections and uncircumcised males were more likely to develop such infections. Patients should be monitored for genital mycotic infections and appropriate treatment should be instituted if these infections occur. For more Drug Warnings (Complete) data for Canagliflozin (14 total), please visit the HSDB record page. Pharmacodynamics This drug increases urinary glucose excretion and decreases the renal threshold for glucose (RTG) in a dose-dependent manner. The renal threshold is defined as the lowest level of blood glucose associated with the appearance of detectable glucose in the urine. The end result of canagliflozin administration is increased urinary excretion of glucose and less renal absorption of glucose, decreasing glucose concentration in the blood and improving glycemic control. A note on type 2 diabetes and cardiovascular disease The risk of cardiovascular events in diabetes type 2 is increased due to the damaging effects of diabetes on blood vessels and nerves in the cardiovascular system. In particular, there is a tendency for hyperglycemia to create pro-atherogenic (plaque forming) lesions in blood vessels, leading to various fatal and non-fatal events including stroke and myocardial infarction. Long-term glycemic control has been proven to be effective in the prevention of cardiovascular events such as myocardial infarction and stroke in patients with type 2 diabetes. Canagliflozin (JNJ 28431754) is a selective SGLT2 inhibitor developed for the treatment of type 2 diabetes mellitus (T2DM) [1][2] - Its core mechanism involves blocking SGLT2 in the renal proximal tubules, inhibiting sodium-dependent glucose reabsorption and promoting glycosuria (glucose excretion in urine), thereby reducing blood glucose levels [1][2] - It exerts insulin-independent hypoglycemic effects, making it suitable for T2DM patients with insulin resistance [1] - Additional benefits include weight reduction (via caloric loss from glycosuria) and blood pressure lowering (via natriuresis and diuresis) [1] - High selectivity for SGLT2 minimizes gastrointestinal side effects associated with SGLT1 inhibition (e.g., diarrhea) [2] - Approved for oral administration in T2DM, with a once-daily dosing regimen supported by its long elimination half-life [2] |
| 分子式 |
C24H25FO5S
|
|
|---|---|---|
| 分子量 |
444.52
|
|
| 精确质量 |
444.14
|
|
| 元素分析 |
C, 64.85; H, 5.67; F, 4.27; O, 18.00; S, 7.21
|
|
| CAS号 |
842133-18-0
|
|
| 相关CAS号 |
Canagliflozin hemihydrate; 928672-86-0; Canagliflozin-d4; 1997338-61-0; Canagliflozin-d6
|
|
| PubChem CID |
24812758
|
|
| 外观&性状 |
White solid powder
|
|
| 密度 |
1.4±0.1 g/cm3
|
|
| 沸点 |
642.9±55.0 °C at 760 mmHg
|
|
| 熔点 |
68-72
|
|
| 闪点 |
342.6±31.5 °C
|
|
| 蒸汽压 |
0.0±2.0 mmHg at 25°C
|
|
| 折射率 |
1.639
|
|
| LogP |
5.34
|
|
| tPSA |
118.39
|
|
| 氢键供体(HBD)数目 |
4
|
|
| 氢键受体(HBA)数目 |
7
|
|
| 可旋转键数目(RBC) |
5
|
|
| 重原子数目 |
31
|
|
| 分子复杂度/Complexity |
574
|
|
| 定义原子立体中心数目 |
5
|
|
| SMILES |
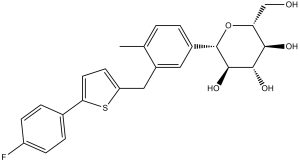
O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1C1C=CC(C)=C(CC2=CC=C(C3C=CC(F)=CC=3)S2)C=1
|
|
| InChi Key |
XTNGUQKDFGDXSJ-ZXGKGEBGSA-N
|
|
| InChi Code |
InChI=1S/C24H25FO5S/c1-13-2-3-15(24-23(29)22(28)21(27)19(12-26)30-24)10-16(13)11-18-8-9-20(31-18)14-4-6-17(25)7-5-14/h2-10,19,21-24,26-29H,11-12H2,1H3/t19-,21-,22+,23-,24+/m1/s1
|
|
| 化学名 |
(2S,3R,4R,5S,6R)-2-[3-[[5-(4-fluorophenyl)thiophen-2-yl]methyl]-4-methylphenyl]-6-(hydroxymethyl)oxane-3,4,5-triol
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.62 mM) (饱和度未知) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
*生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.62 mM) (饱和度未知) in 5% DMSO + 95% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (4.68 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: ≥ 2.08 mg/mL (4.68 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100μL 20.8mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 5 中的溶解度: ≥ 2.08 mg/mL (4.68 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL 澄清 DMSO 储备液加入900 μL 玉米油中,混合均匀。 配方 6 中的溶解度: ≥ 0.5 mg/mL (1.12 mM) (饱和度未知) in 1% DMSO 99% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 7 中的溶解度: 0.5% CMC+0.25% Tween 80 : 18 mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2496 mL | 11.2481 mL | 22.4962 mL | |
| 5 mM | 0.4499 mL | 2.2496 mL | 4.4992 mL | |
| 10 mM | 0.2250 mL | 1.1248 mL | 2.2496 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
The Efficacy and Safety of Sodium-glucose Cotransporter 2 Inhibitors in Patients With Acute Kidney Disease
CTID: NCT06528405
Phase: Phase 2 Status: Not yet recruiting
Date: 2024-08-01