| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
|
| 药代性质 (ADME/PK) |
Metabolism / Metabolites
Incubation of alpha-cedrol and caryophyllene oxide with Neurospora crassa /identified/ 12beta-hydroxy cedrol, 10alpha-hydroxycedrol, and 3beta-hydroxy cedrol, and 12beta-hydroxy caryophyllene oxide as major metabolites, respectively. The antibacterial and radical scavenging activities of the metabolites were evaluated in vitro using broth microdilution and bioauthographic techniques. However, no significant antibacterial and antioxidant activities were observed ... Microbial transformation of (+)-cedrol was investigated by using Staphylococcus epidermidis and found that stereospecific hydroxylation of (+)-cedrol occurred at the C-3 position to form (+)-(3S)-3-hydroxycedrol. |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Cedrol forms colorless crystals. It is found in the wood of cypresses and cedars such as Cedrus atlantica, Cupressus sempervirens, and Juniperus virginiana. It is used in fragnances and as a flavor ingredient in foods and traditional medicine. HUMAN EXPOSURE AND TOXICITY: In an exposure study, odorized and blank air was presented to 26 healthy adult volunteers. A constant concentration of cedrol was presented for 10 minutes with 8 minute blank air intervals. Cedrol caused a relaxant effect with decreased heart rate, respiratory rate, systolic and diastolic blood pressure and increased baroreflex activity. Parasympathetic activity was increased and sympathetic activity was decreased. In another exposure study, a maximization test was carried out with 8% cedrol in petrolatum on 25 male volunteers. Sensitization reactions were observed in 2/25 volunteers. In a pre-test for a human maximization study, no irritation was observed to 8% cedrol, when applied for 48 hr under occlusion on five volunteers. In another study, Pyrolae herba (PHVO) was evaluated for antiproliferative activity against human chondrosarcoma cells. A total of 12 components in PHVO were identified. The major compounds included cedrol (17.08%). PHVO demonstrated potent antitumor activity against SW1353 cells, suggesting its potential use as a therapeutic agent in the treatment of chondrosarcoma. In another study, the aim was to investigate the inhibitory effects of cedrol on the activities of eight major human cytochrome P-450 (CYP) enzymes to assess potential cedrol drug interactions. Cedrol, was found to be a potent competitive inhibitor of CYP2B6-mediated bupropion hydroxylase with inhibition constant (Ki) values of 0.9 uM, comparable with that of a selective CYP2B6 inhibitor, thioTEPA (Ki, 2.9 uM). Cedrol also markedly inhibited CYP3A4-mediated midazolam hydroxylation with a Ki value of 3.4 uM, whereas beta-cedrene moderately blocked CYP3A4. Cedrol at 100 microM negligibly inhibited CYP1A2, CYP2A6, and CYP2D6 activities. Cedrol weakly inhibited CYP2C8, CYP2C9, and CYP2C19 activities, but beta-cedrene did not. These in vitro results indicate that cedrol should be examined for potential pharmacokinetic drug interactions in vivo due to their potent inhibition of CYP2B6 and CYP3A4. ANIMAL STUDIES: A 28-day oral toxicity study was conducted to evaluate toxicity of cedrol in rats. Sixty rats were randomly divided into five groups (10 males or 10 females per group) and a control group of 10 animals. Approximately 0.169% w/v of cedrol was administered at dose of 8.4 mg/kg/day in 20 rats (10/sex) seven days per week via gavage to all the animals for 30 days. Crooked incisors and a swollen mouth were observed in one male rat on day 28. Decrease in absolute brain weight and brain-and ovary-to body weight was observed in female rats. However, these findings were not consistent between the sexes and absence of correlative clinical changes made the consideration of these non-adverse findings of limited toxicological significance. Open epicutaneous tests were carried out in outbred male and female guinea pigs with 8% cedrol, no sensitization reactions were observed. In another study on the sedative effects of cedrol, rats and mice were exposed at 1.0 liter/ minute for 30 minutes. Cumulative spontaneous motor activity was found to be significantly decreased in the cedrol exposed group. Non-Human Toxicity Values LD50 Rabbit Dermal > 5g/kg |
| 参考文献 |
|
| 其他信息 |
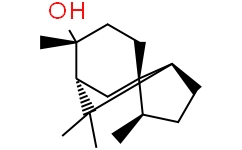
Cedrol is a cedrane sesquiterpenoid and a tertiary alcohol.
Cedrol has been reported in Mappianthus iodoides, Basella alba, and other organisms with data available. Therapeutic Uses Cedrol, beta-cedrene, and thujopsene are bioactive sesquiterpenes found in cedar essential oil and exert antiseptic, anti-inflammatory, antispasmodic, tonic, astringent, diuretic, sedative, insecticidal, and antifungal activities. These compounds are used globally in traditional medicine and cosmetics. /Traditional use/ |
| 分子式 |
C15H26O
|
|---|---|
| 分子量 |
222.372
|
| 精确质量 |
222.198
|
| CAS号 |
77-53-2
|
| PubChem CID |
65575
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.0±0.1 g/cm3
|
| 沸点 |
277.2±8.0 °C at 760 mmHg
|
| 熔点 |
55-59 °C(lit.)
|
| 闪点 |
115.5±10.9 °C
|
| 蒸汽压 |
0.0±1.3 mmHg at 25°C
|
| 折射率 |
1.519
|
| LogP |
4.77
|
| tPSA |
20.23
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
1
|
| 可旋转键数目(RBC) |
0
|
| 重原子数目 |
16
|
| 分子复杂度/Complexity |
321
|
| 定义原子立体中心数目 |
5
|
| SMILES |
C[C@@H]1CC[C@@H]2[C@]13CC[C@@]([C@H](C3)C2(C)C)(C)O
|
| InChi Key |
SVURIXNDRWRAFU-OGMFBOKVSA-N
|
| InChi Code |
InChI=1S/C15H26O/c1-10-5-6-11-13(2,3)12-9-15(10,11)8-7-14(12,4)16/h10-12,16H,5-9H2,1-4H3/t10-,11+,12-,14-,15+/m1/s1
|
| 化学名 |
(3R-(3alpha,3Abeta,6alpha,7beta,8aalpha))-octahydro-3,6,8,8-tetramethyl-1H-3a,7-methanoazulen-6-ol
|
| 别名 |
Eudesmol Cedrol AI3-02178
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~110 mg/mL (~494.67 mM)
H2O : < 0.1 mg/mL |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 2.75 mg/mL (12.37 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
例如,若需制备1 mL的工作液,可将100 μL 27.5 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.75 mg/mL (12.37 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 27.5mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.75 mg/mL (12.37 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.4970 mL | 22.4850 mL | 44.9701 mL | |
| 5 mM | 0.8994 mL | 4.4970 mL | 8.9940 mL | |
| 10 mM | 0.4497 mL | 2.2485 mL | 4.4970 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
|
|