| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| Other Sizes |
| 靶点 |
β-lactam
|
|---|---|
| 体外研究 (In Vitro) |
五水合头孢他啶(0-8 µg/mL,约,24 小时)显示了针对铜绿假单胞菌菌株的抗菌和抗生物膜特性[2]。
五水合头孢他啶(约 0–40 µg/mL,约;18) –20 h) 对嗜麦芽糖链球菌分离株具有抑制作用[3]。 |
| 体内研究 (In Vivo) |
在小鼠大腿感染模型中,头孢他啶(注射液2 h输注,2 000 mg,每8 h一次,持续24 h)五水合物可适度降低细菌密度[4]。
|
| 细胞实验 |
细胞系:铜绿假单胞菌菌株(PAO1、PA1、PA2)
浓度:大约 0-8 µg/mL 孵育时间:24 小时 结果:证明 MIC 值为 2-4 µg/mL抗菌和抗生物膜活性。 |
| 动物实验 |
Animal Model: Murine thigh infection model[4]
Dosage: 2000 mg Administration: 2 h infusion of injection, every 8 h for 24 h. Result: decreased bacterial density when compared to the isogenic strain of NDM (New Delhi metallo-β-lactamase). |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Limited information indicates that ceftazidime produces low levels in milk that are not expected to cause adverse effects in breastfed infants. Avibactam has not been studied in nursing mothers. Occasionally disruption of the infant's gastrointestinal flora, resulting in diarrhea or thrush have been reported with cephalosporins, but these effects have not been adequately evaluated. Ceftazidime-avibactam is acceptable in nursing mothers. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. ◉ Summary of Use during Lactation Limited information indicates that ceftazidime produces low levels in milk that are not expected to cause adverse effects in breastfed infants. Occasionally disruption of the infant's gastrointestinal flora, resulting in diarrhea or thrush have been reported with cephalosporins, but these effects have not been adequately evaluated. Ceftazidime and is acceptable in nursing mothers. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. |
| 参考文献 |
|
| 其他信息 |
Ceftazidime pentahydrate is a hydrate that is the pentahydrate of ceftazidime, a cephalosporin having 7beta-[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-{[(2-carboxypropan-2-yl)oxy]imino}acetyl]amino and 3-pyridinium-1-ylmethyl side-groups. It contains a ceftazidime.
Semisynthetic, broad-spectrum antibacterial derived from CEPHALORIDINE and used especially for Pseudomonas and other gram-negative infections in debilitated patients. |
| 精确质量 |
546.099
|
|---|---|
| 元素分析 |
C, 41.51; H, 5.07; N, 13.20; O, 30.16; S, 10.07
|
| CAS号 |
78439-06-2
|
| 相关CAS号 |
Ceftazidime;72558-82-8
|
| PubChem CID |
6536864
|
| 外观&性状 |
White to off-white solid powder
|
| 熔点 |
>150ºC(dec.)
|
| LogP |
-2.84
|
| tPSA |
244.76
|
| 氢键供体(HBD)数目 |
8
|
| 氢键受体(HBA)数目 |
17
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
42
|
| 分子复杂度/Complexity |
1020
|
| 定义原子立体中心数目 |
2
|
| SMILES |
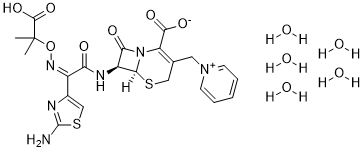
CC(C)(C(=O)O)O/N=C(/C1=CSC(=N1)N)\C(=O)N[C@H]2[C@@H]3N(C2=O)C(=C(CS3)C[N+]4=CC=CC=C4)C(=O)[O-]
|
| InChi Key |
NMVPEQXCMGEDNH-CYWOSJMDSA-N
|
| InChi Code |
InChI=1S/C22H22N6O7S2.5H2O/c1-22(2,20(33)34)35-26-13(12-10-37-21(23)24-12)16(29)25-14-17(30)28-15(19(31)32)11(9-36-18(14)28)8-27-6-4-3-5-7-27/h3-7,10,14,18H,8-9H2,1-2H3,(H4-,23,24,25,29,31,32,33,34)5*1H2/b26-13-/t14-,18-/m0...../s1
|
| 化学名 |
(6S,7S)-7-((Z)-2-(2-aminothiazol-4-yl)-2-(((2-carboxypropan-2-yl)oxy)imino)acetamido)-8-oxo-3-(pyridin-1-ium-1-ylmethyl)-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate
pentahydrate
|
| 别名 |
Ceftazidime; Anhydrous, Ceftazidime;Ceftazidime; Ceftazidime Anhydrous; Ceftazidime Pentahydrate; Fortaz; Fortum; GR 20263; GR-20263; GR20263; LY 139381; LY-139381; LY139381; Pentahydrate, Ceftazidime; Tazidime;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : 2~100 mg/mL (3.65~157.07 mM)
Water : ~25 mg/mL |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 2.5 mg/mL (3.93 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (3.93 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
|
|