| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1g |
|
||
| 5g |
|
||
| 10g | |||
| Other Sizes |
| 靶点 |
β-lactam
|
|---|---|
| 体外研究 (In Vitro) |
针对铜绿假单胞菌菌株,头孢他啶(0–8 μg/mL,约 24 小时)表现出抗菌和抗生物膜特性[2]。
头孢他啶在浓度为 0– 时对嗜麦芽杆菌菌株具有抑制作用。 40 μg/mL,大约 18-20 小时[3]。 |
| 体内研究 (In Vivo) |
在小鼠大腿感染模型中,头孢他啶(注射液输注2 h,每8 h 2 000 mg,持续24 h)可适度降低细菌密度[4]。
|
| 细胞实验 |
细胞系:铜绿假单胞菌菌株 (PAO1、PA1、PA2)
浓度:约 0-8 µg/mL 孵育时间:24 小时 结果:显示 MIC 值为 2-4 µg/mL抗菌和抗生物膜活性。 |
| 动物实验 |
Animal Model: Murine thigh infection model[4]
Dosage: 2000 mg Administration: 2 h infusion of injection, every 8 h for 24 h. Result: decreased bacterial density when compared to the isogenic strain of NDM (New Delhi metallo-β-lactamase). |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Ceftazidime administered intravenously in healthy males produced mean Cmax values of between 42 and 170 μg/mL for doses between 500 mg and 2 g, and are reached immediately following the end of the infusion period. The Cmax for 1 g of ceftazidime administered intramuscularly is attained approximately one hour following injection and is between 37 and 43 mg/L. Following intramuscular administration of 500 mg and 1 g of ceftazidime, the serum concentration remained above 4 μg/mL for six and eight hours, respectively. Ceftazidime Cmax and AUC show linear proportionality to the dose over the therapeutic range. In individuals with normal renal function, ceftazidime given intravenously every eight hours for 10 days as either 1 or 2 g doses showed no accumulation. Approximately 80% to 90% of an intramuscular or intravenous dose of ceftazidime is excreted unchanged by the kidneys over a 24-hour period. When administered intravenously, 50% of the dose appears in the urine within two hours, with another 32% of the dose appearing by eight hours post-administration. Ceftazidime has a volume of distribution of 15-20 L. The mean renal clearance of ceftazidime in healthy subjects ranges from 72 to 141 mL/min while the calculated plasma clearance is approximately 115 mL/min. Metabolism / Metabolites Ceftazidime is not appreciably metabolized. Biological Half-Life Ceftazidime has an elimination half-life of 1.5-2.8 hours in healthy subjects. As ceftazidime is primarily renally excreted, its half-life is significantly prolonged in patients with renal impairment. In patients with creatinine clearance < 12 mL/min, the half-life is prolonged to between 14 and 30 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Limited information indicates that ceftazidime produces low levels in milk that are not expected to cause adverse effects in breastfed infants. Avibactam has not been studied in nursing mothers. Occasionally disruption of the infant's gastrointestinal flora, resulting in diarrhea or thrush have been reported with cephalosporins, but these effects have not been adequately evaluated. Ceftazidime-avibactam is acceptable in nursing mothers. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. ◉ Summary of Use during Lactation Limited information indicates that ceftazidime produces low levels in milk that are not expected to cause adverse effects in breastfed infants. Occasionally disruption of the infant's gastrointestinal flora, resulting in diarrhea or thrush have been reported with cephalosporins, but these effects have not been adequately evaluated. Ceftazidime and is acceptable in nursing mothers. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Ceftazidime plasma protein binding ranges from 5-22.8% (typically less than 10%) and is independent of concentration. Ceftazidime has been shown to bind human serum albumin. |
| 参考文献 |
|
| 其他信息 |
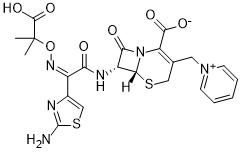
Ceftazidime is a third-generation cephalosporin antibiotic bearing pyridinium-1-ylmethyl and {[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-{[(2-carboxypropan-2-yl)oxy]imino}acetamido groups at positions 3 and 7, respectively, of the cephem skeleton. It has a role as an antibacterial drug, an EC 2.4.1.129 (peptidoglycan glycosyltransferase) inhibitor and a drug allergen. It is a cephalosporin and an oxime O-ether. It is a conjugate acid of a ceftazidime(1-).
Bacteria possess a cell wall comprising a glycopeptide polymer commonly known as peptidoglycan, which is synthesized and remodelled through the action of a family of enzymes known as "penicillin-binding proteins" (PBPs). β-lactam antibiotics, including cephalosporins, are PBP inhibitors that, through inhibition of essential PBPs, result in impaired cell wall homeostasis, loss of cell integrity, and ultimately bacterial cell death. Ceftazidime is a third-generation cephalosporin with broad-spectrum antibacterial activity, including against some treatment-resistant bacteria such as Pseudomonas aeruginosa. Ceftazidime was approved by the FDA on July 19, 1985, and is currently available either alone or in combination with the non-β-lactam β-lactamase inhibitor [avibactam] to treat a variety of bacterial infections. Ceftazidime has been reported in Apis cerana with data available. Ceftazidime is a beta-lactam, third-generation cephalosporin antibiotic with bactericidal activity. Ceftazidime binds to and inactivates penicillin-binding proteins (PBP) located on the inner membrane of the bacterial cell wall. PBPs participate in the terminal stages of assembling the bacterial cell wall, and in reshaping the cell wall during cell division. Inactivation of PBPs interferes with the cross-linkage of peptidoglycan chains necessary for bacterial cell wall strength and rigidity. This results in the weakening of the bacterial cell wall and causes cell lysis. Compared to the second and first generation cephalosporins, ceftazidime is more active against gram-negative bacteria and less active against gram-positive bacteria. Ceftazidine also crosses the blood-brain barrier and reaches therapeutic concentrations in the central nervous system (CNS). Ceftazidime Anhydrous is an anhydrous form of ceftazidime, a third-generation, beta-lactam, cephalosporin antibiotic with bactericidal activity. Semisynthetic, broad-spectrum antibacterial derived from CEPHALORIDINE and used especially for Pseudomonas and other gram-negative infections in debilitated patients. Drug Indication Ceftazidime is indicated for the treatment of lower respiratory tract infections, skin and skin structure infections, urinary tract infections, bacterial septicemia, bone and joint infections, gynecologic infections, intra-abdominal infections (including peritonitis), and central nervous system infections (including meningitis) caused by susceptible bacteria. Ceftazidime is indicated in combination with [avibactam] to treat infections caused by susceptible Gram-negative organisms, including complicated intra-abdominal infections (cIAI), in conjunction with [metronidazole], and complicated urinary tract infections (cUTI), including pyelonephritis, in patients aged three months and older. This combination is also indicated to treat hospital-acquired and ventilator-associated bacterial pneumonia (HABP/VABP) in patients aged 18 years and older. In all cases, to mitigate the risk of bacterial resistance and preserve clinical efficacy, ceftazidime should only be used for infections that are confirmed or strongly suspected to be caused by susceptible bacterial strains. FDA Label Mechanism of Action The bacterial cell wall, which is located at the periphery of Gram-positive bacteria and within the periplasm of Gram-negative bacteria, comprises a glycopeptide polymer synthesized through cross-linking of glycans to peptide stems on alternating saccharides, which is known commonly as peptidoglycan. Cell wall formation, recycling, and remodelling require numerous enzymes, including a family of enzymes with similar active site character despite distinct and sometimes overlapping roles as carboxypeptidases, endopeptidases, transpeptidases, and transglycosylases, known as "penicillin-binding proteins" (PBPs). The number of PBPs differs between bacteria, in which some are considered essential and others redundant. In general, inhibition of one or more essential PBPs results in impaired cell wall homeostasis, loss of cell integrity, and is ultimately bactericidal. Ceftazidime is a semisynthetic third-generation cephalosporin with broad activity against numerous Gram-negative and some Gram-positive bacteria. Like other β-lactam antibiotics, ceftazidime exhibits its bactericidal effect primarily through direct inhibition of specific PBPs in susceptible bacteria. _In vitro_ experiments in Gram-negative bacteria such as _Escherichia coli_, _Pseudomonas aeruginosa_, _Acinetobacter baumannii_, and _Klebsiella pneumoniae_ suggest that ceftazidime primarily binds to PBP3, with weaker binding to PBP1a/1b and PBP2 as well; although binding to other PBPs, such as PBP4, is detectable, the concentrations required are much greater than those achieved clinically. Similarly, ceftazidime showed binding to _Staphylococcus aureus_ PBP 1, 2, and 3 with a much lower affinity for PBP4. Recent data for _Mycobacterium abcessus_ suggest that ceftazidime can inhibit PonA1, PonA2, and PbpA at intermediate concentrations. |
| 精确质量 |
546.099
|
|---|---|
| 元素分析 |
C, 48.34; H, 4.06; N, 15.38; O, 20.49; S, 11.73
|
| CAS号 |
72558-82-8
|
| 相关CAS号 |
Ceftazidime pentahydrate;78439-06-2
|
| PubChem CID |
5481173
|
| 外观&性状 |
White to off-white solid powder
|
| 熔点 |
103-113
|
| LogP |
-2.84
|
| tPSA |
244.76
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
12
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
37
|
| 分子复杂度/Complexity |
1020
|
| 定义原子立体中心数目 |
2
|
| SMILES |
S1C([H])([H])C(C([H])([H])[N+]2C([H])=C([H])C([H])=C([H])C=2[H])=C(C(=O)[O-])N2C([C@@]([H])([C@]12[H])N([H])C(/C(/C1=C([H])SC(N([H])[H])=N1)=N\OC(C(=O)O[H])(C([H])([H])[H])C([H])([H])[H])=O)=O
|
| InChi Key |
ORFOPKXBNMVMKC-LGJNPRDNSA-N
|
| InChi Code |
InChI=1S/C22H22N6O7S2/c1-22(2,20(33)34)35-26-13(12-10-37-21(23)24-12)16(29)25-14-17(30)28-15(19(31)32)11(9-36-18(14)28)8-27-6-4-3-5-7-27/h3-7,10,14,18H,8-9H2,1-2H3,(H4-,23,24,25,29,31,32,33,34)/b26-13+
|
| 化学名 |
(6R,7R)-7-[[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-(2-carboxypropan-2-yloxyimino)acetyl]amino]-8-oxo-3-(pyridin-1-ium-1-ylmethyl)-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate
|
| 别名 |
Fortaz; Fortum; GR 20263; GR-20263; GR20263; LY 139381; LY-139381; LY139381; Tazidime; Ceftazidime anhydrous; Ceftazidime Pentahydrate;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O : 25 ~100 mg/mL (~182.96 mM)
DMSO : ~2 mg/mL ( ~3.65 mM ) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (3.81 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (3.81 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (3.81 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 10% DMSO+40% PEG300+5% Tween-80+45% Saline: ≥ 2.08 mg/mL (3.81 mM) 配方 5 中的溶解度: 100 mg/mL (182.96 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02837835 | COMPLETED | Drug: ceftazidime Drug: ceftazidime |
Pneumonia | CHU de Reims | 2005-03 | Phase 3 |
| NCT03881800 | COMPLETED | Other: titration- blood sample | Ceftazidime Treatment Burned Children |
Assistance Publique - Hôpitaux de Paris | 2020-02-19 | |
| NCT01644643 | COMPLETEDWITH RESULTS | Drug: Ceftazidime - Avibactam ( CAZ-AVI) Drug: Best Available Therapy Drug: Metronidazole |
Complicated Intra-abdominal Infection Complicated Urinary Tract Infection |
Pfizer | 2013-01 | Phase 3 |
| NCT01784445 | COMPLETED | Drug: Ceftazidime | Pancreatitis | University Hospital Rijeka | 2013-06 | Phase 4 |
| NCT03634904 | UNKNOWN STATUS | Drug: Drug bood sampling | Ceftazidime Bacterial Infections Renal Failure Chronic Requiring Hemodialysis |
Centre Hospitalier Universitaire de Charleroi | 2018-09-15 | Not Applicable |
|
|
|